To evaluate nitric oxide (NO) and asymmetric dimethylarginine (ADMA) levels in men with premature ejaculation (PE), which is a common condition that adversely affects quality of life.
Material and methodOf the 20–50-year old men presenting to the urology clinic, who were married or had regular sexual intercourse, 40 that were diagnosed with lifelong PE according to the Premature Ejaculation Diagnostic Tool (PEDT) and intravaginal ejaculation latency time (IELT) measured by a stopwatch were included in the study. The results of the PE group were compared to those of the control group formed with 40 healthy hospital personnel. Venous blood samples were centrifuged and stored at −80°C. The NO and ADMA values were compared between the individuals with and without PE.
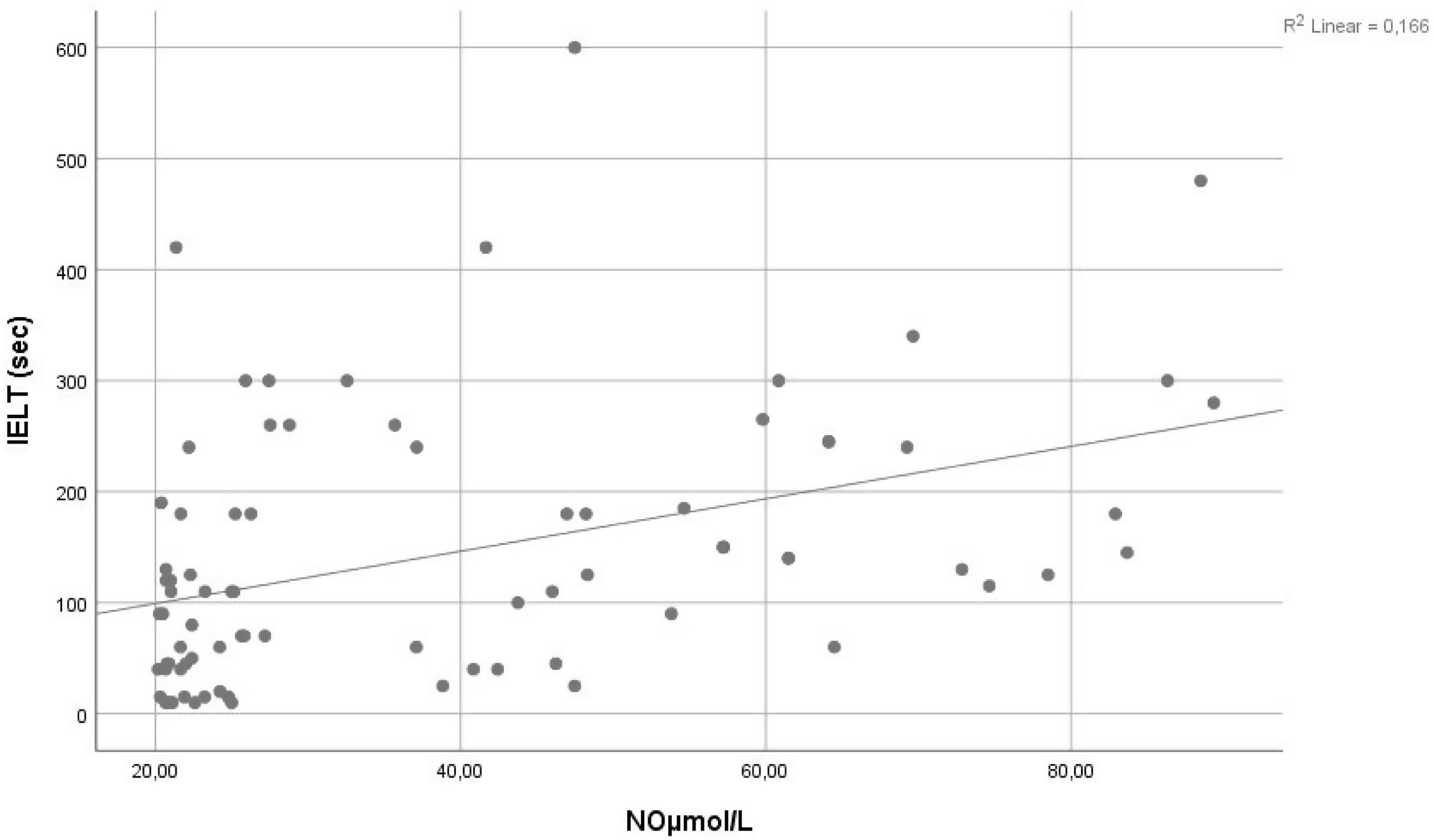
ResultsThere was no statistically significant difference between the groups in terms of age, body mass index (BMI), and The International Erectile Dysfunction Index-5 (IIEF-5) questionnaire scores. The NO and ADMA values were significantly lower in the PE group than in the control group (29.76±13.26μmol/L vs. 48.27±22.71μmol/L; p<0.001 and 1.01±0.49nmol/ml vs. 1.83±1.06nmol/ml; p<0.001, respectively). There was a significant correlation between IELT and NO levels (r=0.407, p=0.001).
ConclusionOur study can contribute to the explanation of the pathophysiology of PE having unclear etiology and treatment. Further studies on these molecules with larger case series are required for the diagnosis and treatment of PE.
Evaluar los niveles de óxido nítrico (NO) y dimetilarginina asimétrica en hombres con eyaculación precoz (EP), que es una afección común que afecta negativamente la calidad de vida.
Material y métodoDe los hombres de 20 a 50 años que acudieron a la clínica de urología, que estaban casados o tenían relaciones sexuales regulares, se incluyeron en el estudio 40 que fueron diagnosticados con EP de por vida según la Herramienta de Diagnóstico de la Eyaculación Precoz y el tiempo de latencia de la eyaculación intravaginal medidos por un cronómetro. Se compararon los resultados del grupo de EP con los del grupo control formado por 40 personas sanas del hospital. Las muestras de sangre venosa se centrifugaron y almacenaron a −80°C. Se compararon los valores de NO y dimetilarginina asimétrica entre los individuos con y sin EP.
ResultadosNo hubo diferencias estadísticamente significativas entre los grupos en términos de edad, índice de masa corporal y puntajes del cuestionario International Erectile Dysfunction Index-5. Los valores de NO y dimetilarginina asimétrica fueron significativamente más bajos en el grupo de EP que en el grupo control (29,76±13,26 frente a 48,27±22,71μmol/l [p<0,001] y 1,01±0,49 frente a 1,83±1,06nmol/ml [p<0,001], respectivamente). Hubo una correlación significativa entre los niveles de tiempo de latencia de la eyaculación intravaginal y NO (r=0,407, p=0,001).
ConclusiónNuestro estudio puede contribuir a la explicación de la fisiopatología de la EP de etiología y tratamiento poco claros. Se requieren más estudios sobre estas moléculas con series de casos más grandes para el diagnóstico y tratamiento de la EP.
Although the prevalence of lifelong premature ejaculation (PE) varies according to geographical regions, it is reported to be 4 to 39% in large-scale studies. Negatively affecting the quality of life of both the men and their partners, PE is considered to be an important sexual dysfunction with a yet-to-be-elucidated pathology, and thus unclear treatment.1–4 The International Society of Sexual Medicine defines lifelong PE as “a male sexual dysfunction characterized by ejaculation which always or nearly always occurs prior to or within about one minute of vaginal penetration, and the inability to delay ejaculation on all or nearly all vaginal penetrations, and negative personal consequences, such as distress, bother, frustration and/or the avoidance of sexual intimacy”.5
The ejaculator reflex is controlled by a complex interaction of serotonergic, dopaminergic, cholinergic, adrenergic, nitrergic, oxytocinergic and GABAergic neurons. Selective serotonin reuptake inhibitors (SSRIs) have been used for the treatment of PE for the last 25 years. The prolongation of the duration of ejaculation by SSRIs was first reported by Segraves in 1993 as a side effect of these drugs, and in 1994, Waldinger conducted the first placebo-controlled study on the use of paroxetine to prolong ejaculation.6,7 Since then, studies have mostly focused on serotonin, investigating its role in the management of ejaculation, and very few reports have been published about other neurotransmitters, such as nitric oxide (NO).
NO, a biological molecule produced by the NO synthase (NOS) enzyme from l-arginine, contributes to various physiological processes, including neurotransmission, regulation of vascular wall tone, immune response, and sperm function.8–11 Studies have also reported that NO is related to sexual behavior. A decrease in sympathetic tone through NO activity in the medial preoptic area has been associated with the inhibition of ejaculation.12 It has also been reported that NO produced by penile nerves affects erection and circulating NO levels may be associated with erectile dysfunction (ED).13 Symmetric and asymmetric dimethylarginine (ADMA) are derived from methylated proteins.14 ADMA is a competitive inhibitor of all three isoforms of NOS. Elevated ADMA values may lead to a reduction in NO levels and the development of cardiovascular events.15 It has been shown that the increase in ADMA levels may be related to ED through a similar mechanism.16
Previously, Otunctemur et al. compared the PE group with the healthy control group in terms of the NO levels and showed that the NO level was significantly lower in the PE group, and SSRI treatment increased intravaginal ejaculation latency time (IELT) and NO values.17 In the current study, we investigated the role of NO and ADMA in the pathophysiology of PE. To our knowledge, this is the first study in the literature to examine ADMA and NO together in patients with PE.
Material and methodPrior to the study, the approval of the ethics committee of our university (decision dated 27/11/2018 and numbered 33216249-604.01.02-E.53200) was obtained. Patients who presented to our outpatient clinic with PE complaints between December 1, 2018 and November 1, 2019 were included in the study. Lifelong PE patients were selected for study group according to the Premature Ejaculation Diagnostic Tool (PEDT) and IELT (<120s). The International Erectile Dysfunction Index-5 (IIEF-5) questionnaire was administered to all participants. Individuals that were found to have ED according to this questionnaire (score<22) were excluded (n=18) from the study. All selected patients and control group were sexually active (having sexual intercourse at least once a week) over the last six months. IELT was measured by a stopwatch. Excluded from the study were patients with diabetes (n=8), hypertension (n=11), hyperlipidemia (n=9), coronary artery disease (n=7), renal insufficiency (n=3), chronic vascular disease (n=2), and neurological or psychiatric disorders (n=5), have used or are currently using SSRIs (n=3), smokers (n=14), patients with varicocele and/or a history of varicocelectomy (n=8), and those with a history of hypothyroidism or hyperthyroidism (n=4). After the exclusion, 40 patients who met the study criteria were included in the PE group. A control group was also formed with 40 healthy hospital personnel of 20–50 years old, who have PEDT scores<8 and IELT>120s for comparison purposes. Venous blood samples obtained from all participants between 8a.m. and 10a.m. were immediately centrifuged and stored at −80°C. The NO and ADMA values were measured by the ELISA method using the kits supplied from Shanghai Sunred Biological Technology Co., Ltd. The NO levels were determined by measuring the nitrite accumulation in the serum using the Griess reaction with sodium nitrate. The NO and ADMA values were compared between the individuals with and without PE.
Statistical analysisFor the statistical analysis, IBM SPSS Statistics version 25 Windows package program was used. The compliance of data to the normal distribution curve was evaluated by the Shapiro–Wilk test. Student's t-test or the Mann–Whitney U-test was used to compare the continuous variables between the two groups, depending on whether the statistical hypotheses were fulfilled. The relationships between the variables were analyzed by the Pearson or Spearman correlation analysis according to the distribution type of parameters. A p value of <0.05 was considered to be statistically significant.
ResultsThe mean age was calculated as 35.4±7.6 (23–49) years for the PE group and 35.9±5.4 (26–49) years for the control group. No statistically significant difference was found between the two groups in relation to age, BMI, and IIEF-5 questionnaire scores (Table 1). The PEDT questionnaire score was 17.6±1.2 in the PE group and 3.5±1.7 in the control group (p<0.001). IELT was measured as 54.38±34.68 and 233.75±107.05seconds for the PE and control groups, respectively (p<0.001). The NO and ADMA values were significantly lower in the PE group than in the control group (29.76±13.26μmol/L vs. 48.27±22.71μmol/L; p<0.001 and 1.01±0.49nmol/ml vs. 1.83±1.06nmol/ml; p<0.001, respectively). There was a significant correlation between IELT and NO levels (r=0.407, p=0.001) (Fig. 1).
Comparison of the premature ejaculation (PE) and control groups in terms of demographic data and laboratory results.
| PE group(n=40) | Control group(n=40) | p | |
|---|---|---|---|
| Age (years) | 35.40±7.6 | 35.95±5.4 | 0.497 |
| BMI (kg/m2) | 27.22±7.13 | 26.51±3.98 | 0.962 |
| IIEF-5 questionnaire score | 23.60±0.98 | 23.95±1.01 | 0.091 |
| PEDT score | 17.68±1.22 | 3.5±1.73 | <0.001 |
| IELT (s) | 54.38±34.68 | 233.75±107.05 | <0.001 |
| NO (μmol/L) | 29.76±13.26 | 48.27±22.71 | <0.001 |
| ADMA (nmol/ml) | 1.01±0.49 | 1.83±1.06 | <0.001 |
BMI: body mass index, PEDT: Premature Ejaculation Diagnostic Tool, IIEF-5: International Erectile Dysfunction Index-5, IELT: intravaginal ejaculation latency time, NO: nitric oxide, ADMA: asymmetric dimethylarginine.
Many molecules, including NO and serotonin are involved in the physiology of ejaculation.18,19
Omu et al. stated that the NO is one of the most important neurotransmitters in the human reproductive system and decreased NO levels may play a role in the pathophysiology of PE by leading to an increase in cavernous smooth muscle contractions.20 The most important effect of NO is the activation of guanylate cyclase. The synthesis of cyclic guanosine monophosphate (cGMP) causes relaxation and vasodilatation in vascular smooth muscles.21,22 In the central and peripheral nervous systems, the NO-cGMP pathway plays a key role in sexual function.23 During ejaculation, the NO-cGMP pathway is actively involved in the contraction of smooth muscles in seminal vesicles and ductus deferens.24 The relaxation of the smooth muscles in the genitourinary system, especially those associated with vas deferens, has an effect on delaying ejaculation. In vitro experiments have shown that different NO drugs can significantly increase cGMP levels in human seminal vesicle smooth muscles, and thus prevent smooth muscle contraction in seminal vesicles, vas deferens, prostate, and urethra.25 It has also been suggested that drugs that can increase NO can be used in the treatment of PE in the future.
Otunctemur et al. compared the serum NO levels of healthy men with PE patients divided into three subgroups according to the type of SSRI used. Initially, the NO values were significantly lower in the PE groups compared to the healthy group (31.8μmol/L, 30.44μmol/L, and 30.8μmol/L vs. 42.84μmol/L). Following the SSRI treatment applied to the PE groups, there was a significant increase in the NO values (from 31.8μmol/L to 35.8μmol/L, 30.44μmol/L to 38.08μmol/L, and 30.8μmol/L to 36.4μmol/L; p<0.005).17 Similarly, in our study, the serum NO levels of the PE group were significantly lower compared to healthy individuals. Furthermore, Otunctemur reported a significant increase in IELT with the increase in NO. Supporting this finding, we found a positive correlation between NO levels and IELT (Fig. 1). The prolongation of IELT may be associated with the elevated NO values increasing the cGMP levels. Kirecci et al. measured the seminal NO levels and initially found them to be significantly higher in the PE group than in healthy subjects; however, after one month of SSRI treatment, the authors showed a decrease in the seminal NO levels.26 In both these studies (Otunctemur et al. and Kirecci et al.), the NO values in the serum and seminal fluid were affected by SSRI treatment and reached levels similar to those of healthy controls; however, neither author commented on the mechanism by which this improvement occurred. Nevertheless, these two previous studies, as well as the current study indicate the important role of NO in the pathophysiology of PE.
Studies on phosphodiesterase type 5 (PDE5) inhibitors sildenafil and vardenafil have shown that these drugs reduce central and peripheral cGMP degradation and increase intracellular cGMP,27 Which leads to the reduced transmission of sympathetic impulses to the peripheral nervous system, resulting in delayed ejaculation, prolonged IELT, and increased sexual quality by effectively treating PE.28,29 Krishnappa et al. state that PDE5 inhibitors can be used alone or in combination in the treatment of PE.30 With the guidance of these studies, the use of PDE5 inhibitors that increase cGMP may be more commonly used in PE treatment in the future or alternative methods to increase NO in the body can provide effective solutions for the treatment of this condition. This requires further research with larger case series.
In the current study, we also found the serum ADMA levels, which is a NOS inhibitor, to be significantly lower in the PE group compared to the healthy group. To the best of our knowledge, this was the first study to investigate the serum ADMA levels in this patient group. Previously, ADMA levels were reported to be increased in patients with cardiovascular and metabolic diseases, such as hypercholesterolemia, atherosclerosis, hypertension, chronic heart or renal failure, diabetes mellitus, stroke, and hyperhomocysteinemia.31–34 In addition, the presence of a pathophysiological relationship has been noted between vascular endothelial dysfunction, particularly coronary artery disease and ED.35,36 These studies, which generally report vascular pathologies, provide the explanation that increased ADMA inhibits NOS and thus decreases NO synthesis, which results in the reduction of blood flow to organs and causes CAD and ED. In the current study, the patients who were diagnosed with ED according to the IIEF-5 form were excluded in order to eliminate the effect of PE and ED coexistence. There was no significant difference between the groups in terms of the IIEF-5 questionnaire scores. The ADMA levels were significantly lower in the PE group, similar to the case in the NO levels. Since ADMA was not previously investigated in PE patients, we were not able to compare our data to the literature. Generally, there is an inverse correlation between NO and ADMA, while some studies have also observed an increase in NO and ADMA together.37 The simultaneous low detection of both molecules may result from the decreased ADMA levels with the increase in the activity of dimethylarginine dimethylaminohydrolase (DDAH), which metabolizes ADMA, or a compensatory decrease in the ADMA levels due to the reduced NO levels in the body. Studies have shown that DDAH activity is regulated by NO.38,39 This means that when NO production decreases under certain conditions, DDAH activity will increase and ADMA levels will decrease so that the NOS enzyme can be reactivated.40
The limitations of our study include the limited number of patients and the lack of studies in the literature for the comparison of ADMA findings.
ConclusionThe results we reported can contribute to the explanation of the pathophysiology, and thus treatment of PE, which, despite many studies, remain unclear and assist in the diagnosis-treatment process of this important condition that negatively affects the quality of life of men and their partners. Our study may prompt the design of further studies assessing the impact of NO/ADMA in PE pathophysiology.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis research was supported by the Scientific Research Project Unit of Erzincan Binali Yildirim University.
Conflict of interestThere is no conflict of interest of any authors in relation to the submission.