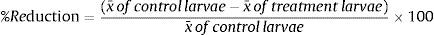The dog acts as a reservoir and environmental disseminator of potentially zoonotic parasites.
AimsThe objective of this work was to study the fungus Monacrosporium thaumasium regarding its nematicidal potential in laboratory trials and its proteolytic profile.
MethodsThe in vitro test was carried out through two assays (A and B). In assay A, conidia of the fungus N34a were added in positive coprocultures for Angiostrongylus vasorum. In assay B, crude extract (treated group) and distilled water (control group) were added to coprocultures. Next, the proteolytic profile of crude extract of the nematophagous fungus M. thaumasium (NF34a) was revealed by performing a zymogram.
ResultsThere was a reduction (p<0.01) in the averages of larvae recovered from the treated groups (conidia and crude extract) in relation to control groups. The zymogram suggested that the nematophagous fungus M. thaumasium produces a protease of approximately 40kDa.
ConclusionsThe results of this work confirm that the conidia as well as the crude extract of the fungus M. thaumasium may be used to control A. vasorum L1. The proteolytic profile suggested the presence of one protease (Mt1) of approximately 40kDa that in the future may be used in biological control of L1 of this nematode.
El perro actúa como reservorio y propagador ambiental de los parásitos potencialmente zoonóticos.
ObjetivosEl objetivo del presente estudio fue examinar el potencial nematicida del hongo Monacrosporium thaumasium en pruebas de laboratorio, al igual que su perfil proteolítico.
MétodosEl examen in vitro se efectuó mediante 2 ensayos (A y B). En el análisis A, se añadieron conidias del hongo N34a a coprocultivos positivos para Angiostrongylus vasorum. En el ensayo B, se añadieron extracto bruto (grupo tratado) y agua destilada (grupo de control) a los coprocultivos. A continuación, se puso de relieve el perfil proteolítico de extracto bruto del hongo nematófago M. thaumasium (NF34a) mediante la realización de un zimograma.
ResultadosSe observó una reducción (p<0,01) en el número medio de larvas recuperadas de los grupos tratados (conidias y extracto bruto) en relación con los grupos de control. El zimograma evidenció que el hongo nematófago M. thaumasium produce una proteasa de aproximadamente 40kDa.
ConclusionesLos resultados del presente estudio confirman que las conidias, así como el extracto bruto del hongo M. thaumasium, pueden utilizarse para el control de A. vasorum L1. El perfil proteolítico mostró la presencia de una proteasa (Mt1) de alrededor de 40kDa que, en el futuro, se puede utilizar en el control biológico de L1 de este nematodo.
Nematophagous fungi have been successfully used on in vitro studies of biological control. These organisms called “eaters of helminths” stand out for their availability and easy maintenance under laboratory conditions. However, after many years, little is known about the real mechanism of their infection versus nematodes.1,13 In this context, researches about the production of primary metabolites, such as proteolytic enzymes, are performed; these enzymes have been studied in biological assays of nematicidal activity.9,17 On the other hand, nematophagous fungi have been pointed out, especially due to the promising results observed in laboratory conditions through the interaction between fungi and the first-stage larvae of Angiostrongylus vasorum.5
Species of the genus Angiostrongylus (Angiostrongylus costaricensis, Angiostrongylus cantonensis and Angiostrongylus vasorum) can cause problems for the definitive host (dogs) and eventually parasitize man.2 The literature has reported some work that have aimed to seek alternative measures, such as biological control, to be used in combat of angiostrongyliasis.
The role of the dog as a reservoir and environmental disseminator of potentially zoonotic parasites was reported long ago. Moreover, it is known that in many places this important aspect of transmission of zoonotic agents is present, especially in poorer regions of Asia, South America, Africa and Australia.18 In Brazil,16 the risk of human infection through contaminated feces of dogs may be significantly higher than in developed countries where a similar prevalence is observed. In this context,15 the fecal environment is the favorable place for the development of helminths that parasitize domestic animals and may also be zoonotic, since in this environment most genera of helminth pass from the egg stage to infective larvae stage. On the other hand, there is a need for studies aimed at discovering the action of substances produced by these fungi in environments that mimic the natural condition of infection, for example, contaminated feces, so they can eventually be used in environmental control of larvae of this nematoda. Among those substances, the use of proteases produced by nematophagous fungi stands out.6
The aim of this work was to study the proteolytic profile of the nematophagous fungus Monacrosporium thaumasium and its activity against A. vasorum larvae.
Materials and methodsOrganismsPositive feces of dogs were used for A. vasorum previously maintained in the Parasitology Department, Federal University of Minas Gerais.14
To obtain a crude extract, it was used an isolate of the nematophagous fungus M. thaumasium (NF34a), isolated from soil in Brazil and maintained through continuous transfer to solid culture media (corn meal agar 2%) in the Laboratory of the Department of Veterinary Parasitology, Federal University of Viçosa. For the production of crude extract, mycelia of this fungus were obtained by transferring culture discs (about 5mm in diameter) to flasks previously autoclaved and containing 50ml of liquid medium. The liquid medium was composed of: glucose (10g/l), casein (10g/l), K2HPO4 (5.0g/l), MgSO4 (0.10g/l), ZnSO4 (0.0050g/l); FeSO4 (0.001g/l) and CuSO4 (0.50mg/l). Next, the flasks containing the fungal inoculum grown in shaker under agitation of 120×g and after six days, the supernatant was collected and filtered using Whatman no. 1 filter paper at 4°C. The methodology for production and obtaining of the crude extract was based on the work of Braga et al.6
AssaysThe in vitro test was carried out through two assays (A and B). In assay A, 1000 conidia of the fungus N34a were added in positive coprocultures for A. vasorum, constituting the treated group. The control group was constituted by coprocultures of positive feces, without fungus. In assay B, 5ml of crude extract produced by NF34a were added to coprocultures, constituting the treated group; coprocultures with only the addition of distilled water (5ml) constituted the control group. In both assays, coprocultures were incubated at 25°C in the dark for 8 days. Six replicates were carried for each assay.
At the end of this period, first-stage larvae (L1) were obtained using the modified method of Baermann in both assays (A and B), and the total amount of larvae was obtained by a simple rule of three.4 Then, data were interpreted by analysis of variance and the efficiency of predation of L1 in relation to the control groups was assessed by the Tukey test at 1% probability.3 Subsequently, the average percentage reduction of larvae was calculated according to the following equation:
Proteolytic profileNext, the proteolytic profile of crude extract of the nematophagous fungus M. thaumasium (NF34a) was revealed by performing a zymogram, using casein as substrate (Casein-SDS-PAGE), as described by Hummel et al.12 with some modifications. The action of the enzyme can be observed by the formation of white halos.
Results and discussionAfter eight days, it was demonstrated in assays A and B that the conidia and the crude extract of M. thaumasium (NF34a) were effective in reducing the number of A. vasorum L1. In addition, at the end of the assays, it was noted a statistical difference (p<0.01) between the means of larvae recovered from the treated groups and those from the control groups with the following percentages: 40% and 52% for the assays A and B, respectively. Via the zymogram performed, it was revealed the proteolytic profile of the crude extract produced by NF34, Fig. 1, where the presence of a halo of digestion of casein can be observed, revealing the presence of a protease of approximately 40kDa.
The soil contaminated with infective stages (eggs and/or larvae) has been appointed as one of the major sources of contamination by geohelminths.10 Fungal structures, such as conidia, have proven to be effective in controlled trials against A. vasorum L1.5,7 On the other hand, the use of crude extract is also a artifice, although recent, that has been used to combat eggs and/or larvae of geohelminths.7,8 In the present work, the fecal environment was mimetized by use of coprocultures and the results were very promising.
In assay A, conidia of the fungus NF34 poured in the coprocultures decreased the number of L1 recovered (40%). This result is interesting and confirms previous reports about the predatory activity of conidia of this fungus after the interaction in culture medium containing agar-water and A. vasorum L1 at the end of seven days (74.5%).5 However, some comparisons can be made between the respective works: (1) in this study coprocultures (poor medium) were utilized to mimic the real condition of contamination, while in the work cited above agar-water was used, which although is also poor in nutrients probably gives a larger pattern of control, especially in relation to contamination by other agents; (2) the agar-water medium routinely used in laboratory tests has not yet presented the results achieved under natural conditions, an important information for the use of fecal material.
In relation to assay B, the results obtained can be compared also with the same report5 of the use of crude extract of the predatory fungus Duddingtonia flagrans on A. vasorum L1. However, even belonging M. thaumasium to the same group of fungi (predators), this information is important because the concentrated crude extract of D. flagrans was evaluated directly on the L1 in a short period of time, 24 and 48 h, demonstrating a percentage reduction of 53.5% and 71.3%, respectively. In the present work, the crude extract of NF34 showed a percentage of reduction of 52% when recovering after eight days L1 larvae obtained from treated coprocultures.
This result suggests that possibly the crude extracts produced by predatory nematophagous fungi contains hydrolytic enzymes such as proteases, which maintains its biological activity even under non-optimal pH, temperature, humidity and salt concentrations. On the other hand, the zymogram demonstrated a proteolytic profile of the crude extract of NF34a that points to the presence of one protease with molecular weight similar to the proteases described from predatory nematophagous fungi.11 Moreover, the zymogram suggested the presence of one protease (Mt1) of approximately 40kDa, which is in according to the recent work of Soares et al.17 These results may eventually contribute to the discovery of new methodologies that can help in environmental decontamination of geohelminths.
The results of this work confirm that the conidia, as well as the crude extract of the fungus M. thaumasium, may be used to control A. vasorum L1. The proteolytic profile suggested the presence of one protease (Mt1) of approximately 40kDa that in the future may be used in biological control of L1 of this nematode.
Authors’ declarationWe declare that: (a) the content of the article is original and was not published previously; (b) there is no conflict of interests, related to financial aspects; (c) all the authors have read and approved this manuscript.
Conflicts of interestAll authors declare no conflict of interest.
The authors would like to thank CNPq, FAPEMIG and CAPES for the financial support and grant concession.