Candida albicans is an opportunistic dimorphic fungus commonly present in the human oral cavity that causes infections in immunocompromised patients. The antigen variability, influenced by growth conditions, is a pathogenicity factor.
AimsTo determine the effect of nutritional and heat stress on the antigen expression of C. albicans, and to identify major antigens recognized by human salivary secretory immunoglobulin A (sIgA).
MethodsUnder various different nutritional conditions, heat shock was induced in C. albicans cells in stationary and exponential growth phases. The expression of protein determinants of C. albicans was assessed by Western blot analysis against human saliva. The antigens were purified and characterized by two-dimensional electrophoresis and identified by protein microsequencing.
ResultsFive antigens recognized by salivary IgA were characterized as mannoproteins due to their reactivity with concanavalin A. They did not show reactivity with anti-heat shock protein monoclonal antibodies. Two of them (42 and 36kDa) were found to be regulated by heat shock and by nutritional stress and they were identified as phosphoglycerate kinase and fructose bisphosphate aldolase, respectively.
ConclusionsThese glycolytic enzymes are major antigens of C. albicans, and their differential expression and recognition by the mucosal immune response system could be involved in protection against oral infection.
Candida albicans es un hongo dimórfico oportunista que, con frecuencia, está presente en la cavidad oral del ser humano donde da lugar a infecciones en pacientes inmunocomprometidos. Influida por las condiciones de crecimiento, la variabilidad antigénica es un factor de patogenicidad.
ObjetivosDeterminar el efecto del estrés nutricional y térmica en la expresión antigénica de C. albicans, e identificar los principales antígenos reconocidos por la inmunoglobulina secretora A (sIgA) salival humana.
MétodosEn diferentes condiciones nutricionales, se indujo un choque térmico en células de C. albicans en fase de crecimiento estacionario y exponencial. La expresión de los determinantes proteicos de C. albicans se analizó mediante inmunotransferencia frente a la saliva humana. Los antígenos se purificaron y caracterizaron mediante electroforesis bidimensional y se identificaron mediante microsecuenciación de proteínas.
ResultadosSe caracterizaron cinco antígenos reconocidos por la IgA salival como manoproteínas debido a su reactividad con la concanavalina A. Ninguno manifestó reactividad con los anticuerpos monoclonales anti-proteína de choque térmico. Se encontró que el choque térmico y el estrés nutricional regulaban dos de ellos (de 42 y 36 kDa) identificados como fosfoglicerato quinasa y fructosa bifosfato aldolasa, respectivamente.
ConclusiónEstas enzimas glucolíticas son antígenos mayores de C. albicans, y su expresión diferencial y el reconocimiento por el sistema de la respuesta inmunitaria de la mucosa podrían participar en la protección frente a las infecciones orales.
Candida albicans is an oral commensal yeast in around a third to a half of the healthy population.8 However, this fungus is the main cause of superficial forms of candidiasis, and clinical observations indicate that mucocutaneous Candida infections are commonly associated with a defective immune response.16
Saliva is an important element of the protective barrier provided by the oral epithelium1 because it contains a variety of proteins, in particular secretory immunoglobulin A (sIgA), which affords protection by inhibiting adherence and penetration into mucosal tissues of microorganisms,14 including C. albicans.19 The sIgA coating of Candida cell walls is observed in clinical smears from patients with oral and vaginal candidiasis. Some studies13,15 have identified antigens of C. albicans that react with salivary sIgA, such as heat shock mannoproteins. They are expressed at higher levels in cells grown at 37°C than in cells grown at 24°C. In this work, we have identified two glycolytic enzymes regulated by heat and nutritional stress as new antigens recognized by antibodies in human saliva.
Materials and methodsMicroorganisms and culture conditionsThree C. albicans strains, UPV1360, VW32 and UPV1413, were used. The strains were grown in Sabouraud Dextrose Broth (SDB) with 4% of glucose at 24°C and stirred at 120rpm, for 9h or 24h to obtain exponential phase or stationary phase cells, respectively. They were harvested by centrifugation (1250×g, 5min), washed twice with sterile 50mM phosphate-buffered saline (PBS, pH 7.4), and then resuspended in one of the following culture media: (i) SDB in which the cells had been growing; (ii) fresh SDB; (iii) fresh SDB without peptone; or (iv) fresh SDB without glucose. All of them were incubated at 37°C for 15, 30, 60 and 120min to study the heat shock response, or at 24°C for 120min as the control.
Saliva collectionWhole saliva from 13 Candida-free healthy individuals (7 men and 6 women; 20–40 years old) was collected with Salivettes (Sarstedt, Barcelona, Spain), after they had signed an informed consent form. Half of the sample volume was pooled and the other half was stored individually. After centrifugation, as described above, supernatants were adsorbed with C. albicans UPV1360 cells grown at 24°C for 24h to eliminate non-specific binding in the Western blot (WB).5
Electrophoresis and Western blot analysis of antigen extractsThe antigen extracts were obtained from 1010C. albicans cells and analyzed by one-dimensional electrophoresis (1-DE) according to the method described by Calcedo et al.5 The two-dimensional electrophoresis (2-DE) was carried out as in Hernando et al.7
The 1-DE and 2-DE gels were transferred to PVDF membranes (Immobilon-P; Millipore, Billerica, MA, USA) and mannoproteins were detected using concanavalin A.7 For WB analysis, adsorbed saliva or anti-Hsp 90 (Bionova, Madrid, Spain), 70, 60, and 25kDa (Sigma, St. Louis, MO, USA) mouse monoclonal antibodies were used as primary antibodies, and goat anti-human IgA and anti-mouse IgG peroxidase (Sigma) as secondary antibodies. Bands were revealed with diaminobenzidine (DAB)5 and analyzed using the Bio Image 50S system with the Whole Band and 2D Analyzer software (Millipore).
Protein purification and microsequencingCrude extract (10mg) was fractionated using the 491 Prep Cell and the Econo System (Bio-Rad, Hercules, CA, USA) in a 28mm diameter, 13% acrylamide/bis-acrylamide gel, at 1ml/min following the manufacturer instructions. Fractions containing the 36kDa or the 42kDa band were recovered, dialysed, concentrated using 10kDa Ultrafree-Biomax membranes (Millipore), and analyzed by 2-DE and WB. The spots were excised from the 2-DE gels and sequenced by Edman degradation (Proteomics Laboratory of the CNB, Cantoblanco, Madrid), and sequences obtained were identified by searching, either in SWISS-PROT, in TrEMBL or using NCBI's Basic Local Alignment Search Tool (BLAST).
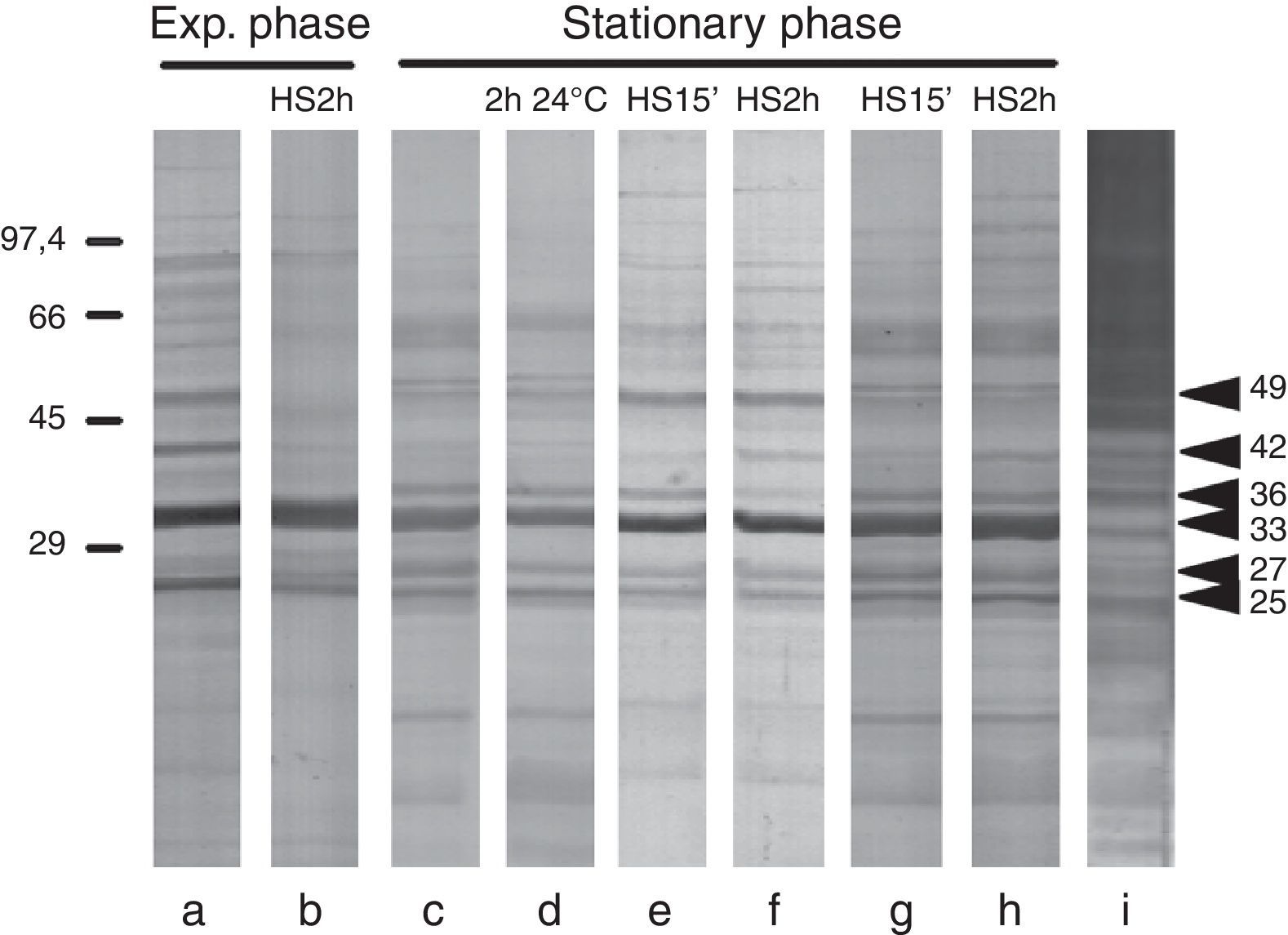
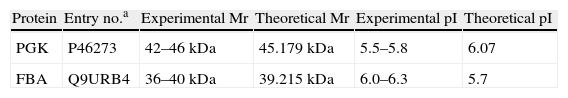
Results and discussionOf all the different types of infection caused by Candida, oral and vaginal candidiasis are the most common clinical presentations,6,12 and the lack of sIgA against Candida could explain the recurrence of candidiasis in some patients.18 Several HSPs of 205, 180, 140 and 110kDa recognized by sIgA have already been identified by our group.5,13,15 In this work, we have found five major antigenic components, 42, 36, 33, 27 and 25kDa (Fig. 1), recognized by salivary sIgA. Two of them, 42 and 36kDa, modified their expression in all the strains under different growth and stress conditions, and commercial anti-HSPs monoclonal antibodies did not react with them, suggesting that they could be fragments that do not contain the recognized epitopes or new HSP. These antigens might be expressed on C. albicans cells colonizing human mucosal surfaces, since in 76.9% and 69.2% of saliva samples there was a reaction with these antigens, respectively (data not shown), although we cannot rule out previous asymptomatic candidiasis in these healthy individuals. Only one of the saliva samples did not react with any of these antigens. The 42kDa and 36kDa antigens were identified as phosphoglycerate kinase (PGK) and fructose bisphosphate aldolase (FBA), respectively (Table 1). Both these antigens are mannoproteins involved in glycolytic metabolism, and therefore their expression is coregulated by nutritional and heat stress.
Western blot of protein extracts from: (a) exponential phase cells; (b) exponential phase cells incubated for 2h at 37°C in the SDB in which cells had been growing; (c) stationary phase cells; (d) stationary phase cells incubated for 2h at 24°C in fresh SDB; (e and f) stationary phase cells incubated for 15min or 2h at 37°C in fresh SDB, respectively; (g and h) stationary phase cells incubated for 15min or 2h at 37°C in SDB without peptone, respectively; and (i) mannoprotein detection using concanavalin A in stationary phase cells incubated for 2h at 37°C in fresh SDB.
Protein identification after sequencing. Experimental and theoretical molecular mass (Mr) and isoelectric point (pI).
| Protein | Entry no.a | Experimental Mr | Theoretical Mr | Experimental pI | Theoretical pI |
| PGK | P46273 | 42–46kDa | 45.179kDa | 5.5–5.8 | 6.07 |
| FBA | Q9URB4 | 36–40kDa | 39.215kDa | 6.0–6.3 | 5.7 |
aEntry number according to the UniProtKB database.
PGK, in optimal nutritional conditions (Fig. 1a), is an immunodominant antigen, but it was not expressed in the stationary growth phase (Fig. 1c) or under conditions of nutritional stress (Fig. 1g and h). However, it was expressed again in stationary phase cells and under starvation conditions after a heat shock (Fig. 1f and h). So, PGK expression seems to need an active metabolic phase or heat shock treatment. This antigen, which has been found in the C. albicans cell wall,2 is an enzyme involved in ATP production,21 and could generate energy for local cell wall biosynthesis as well as for cytoplasmic activity.4 Reactive oxygen species are involved in the transcriptional activation of the PGK1gene. In fact, it has been recently described as a potential protein biomarker of intracellular oxidative status in human colon carcinoma cells.9 In the stationary growth phase, the overexpression of PGK we detected could be related to higher requirements for energy by enhancing ATP production.
In contrast, FBA expression was observed in the stationary phase of growth and under starvation conditions. C. albicans FBA1p belongs to the family of class II aldolases found predominantly in fungi and prokaryotes,10 and it is involved in the formation of the cell wall.3 Further, antibodies against FBA have been detected in serum samples from human, monkeys and mice infected by C. albicans, protozoa or parasitic worms.11 Recently, it was shown that a vaccine combining β-mannan and the FBA peptide conferred immunity against candidiasis.20 On the other hand, considering that FBA1 is a single gene in C. albicans and that it exhibits strong sequence similarity to its orthologues in Schizosaccharomyces pombe, Aspergillus nidulans and Neurospora crassa, an antifungal agent directed against C. albicans FBA1 might have broad specificity.17
In conclusion, at least one of the two glycolytic enzymes of C. albicans, phosphoglycerate kinase and fructose bisphosphate aldolase, whose expression is regulated by nutritional and heat stress, were recognized as immunogenic in the most of the studied human saliva (92.3%) samples. In spite of the fact that continuous contact with C. albicans cells or cross-reactivity with other pathogens might be the origin of these specific sIgAs in healthy individuals, specific sIgAs against these antigens could be useful for controlling Candida growth and, hence, the infection process. In particular, these antigens could be of interest in antifungal therapy and explain, at least in part, the pathogenesis of mucosal C. albicans infection.
This work has been partially supported by a SAIOTEK Grant (S-PE09UN41), a Consolidated Research Group IT343/10 of the Government of the Basque Country, and a grant from the University of the Basque Country (UE 08/14).
We thank Ideas Need Communicating Language Services SL for proofreading the English text.