Lectins are carbohydrate-binding proteins widely distributed in nature. They constitute a highly diverse group of proteins consisting of many different protein families that are, in general, structurally unrelated. In the last few years, mushroom and other fungal lectins have attracted wide attention due to their antitumour, antiproliferative and immunomodulatory activities. The present mini-review provides concise information about recent developments in understanding lectins from human pathogenic fungi. A bibliographic search was performed in the Science Direct and PubMed databases, using the following keywords “lectin”, “fungi”, “human” and “pathogenic”. Lectins present in fungi have been classified; however, the role played by lectins derived from human pathogenic fungi in infectious processes remains uncertain; thus, this is a scientific field requiring more research.
This manuscript is part of the series of works presented at the “V International Workshop: Molecular genetic approaches to the study of human pathogenic fungi” (Oaxaca, Mexico, 2012).
Las lectinas son proteínas que se unen a los hidratos de carbono y están ampliamente distribuidas en la naturaleza. Constituyen un grupo muy diverso de proteínas incluidas en muchas familias que en general carecen de relación estructural. En los últimos años, se ha prestado mucha atención a las lectinas fúngicas debido a sus actividades antitumorales, antiproliferativas e inmunomoduladoras. La presente revisión proporciona información sucinta sobre los progresos recientes acontecidos en la comprensión de estas moléculas a partir de hongos patógenos para el ser humano. Emprendimos una búsqueda bibliográfica de los estudios publicados en las bases de datos Science Direct y PubMed, usando las palabras claves: «lectin» (lectina), «fungi» (hongos), «human» (humano) y «pathogenic» (patogénico). Se han clasificado las lectinas presentes en los hongos; sin embargo, el papel que desempeñan en los procesos infecciosos de hongos patógenos para el ser humano sigue por dilucidar, por lo que este es un ámbito científico que requiere mayor investigación.
Este manuscrito forma parte de la serie de artículos presentados en el «V International Workshop: Molecular genetic approaches to the study of human pathogenic fungi» (Oaxaca, México, 2012).
More than 70,000 species of fungi have been described, and it is likely that many more exist. The known species include yeasts, moulds, rusts, smuts, puffballs, and mushrooms. The study of these eukaryotes has been motivated by their unique and fascinating biology, their many useful products (including wine, cheese, and antibiotics), their utility as experimental systems for basic biology and protein expression, and their relevance as animal and plant pathogens. Fungi are eukaryotic heterotrophs and absorb food from their environment. They are non-motile and have life cycles that incorporate both sexual and asexual reproduction. They typically have elongated filaments or hyphae, which have cell walls that comprise complex polysaccharides including mannans, galactans, glucans, and chitin. There are four major phyla of fungi; each of them is extremely diverse, and fungi are assigned to a phylum on the basis of their mechanism for producing asexual spores. These phyla are the Chytridiomycota (primitive aquatic fungi), Mucoromycotina (e.g., black bread mould, Rhizopus nigricans), Ascomycota (e.g., Saccharomyces, Candida, Aspergillus, Neurospora, and morel mushrooms), and Basidiomycota (e.g., mushrooms, rot fungi, and puffballs). Some fungi are extremely beneficial to humans (e.g., the yeast Saccharomyces, which is used in fermentation) and some are not (e.g., pathogenic yeasts such as species of Candida and Cryptococcus).6
Lectins are a well-known class of multivalent carbohydrate binding proteins of non-immune origin that recognize diverse sugar structures with a high degree of specificity.24 Lectins have been involved in cellular signalling, malignancy, host pathogen interactions, scavenging of glycoproteins from the circulatory system, cell–cell interactions in the immune system, differentiation and protein targeting to cellular compartments.23 Plant and animal lectins are subjected to extensive studies6,19 and very little information is available on lectins from fungi. However, the first fungal lectin, called «lectin phallin», was reported in 1891 by Kobert from Amanita phalloides,12 which was later identified as a haemolytic agent.30 In the last few years, mushroom and other fungal lectins have attracted wide attention due to their antitumour, antiproliferative and immunomodulatory activities.4 More recently, several reports on lectins from lower and pathogenic fungi have shown their physiological role still remains uncertain.30
Fungal lectinsFungal lectins have been isolated from mycelium,14 sporomes,10 conidia,29 basidiomes,9 and fruiting bodies.28 Fungal lectins have been classified according to their structure in the following families:
- 1.
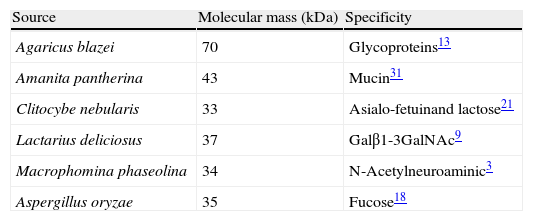
Family resembling β-propeller-fold lectins. β-propellers are toroidal folds in which repeated four-stranded β meanders are arranged in a circular and slightly tilted fashion, like the blades of a propeller, Aleuria aurantia has this folding type, with six blades. This lectin is a dimer of two identical subunits of about 36kDa, each of them binding preferably α1,2 linked fucose residues. A. aurantia lectin binds preferentially to fucose linked (α1,6) to N-acetylglucosamine, or to fucose linked (α1,3) to N-acetyllactosamine related structures.8
- 2.
Family resembling actinoporin-like lectin. Actinoporins are potent eukaryotic pore-forming toxins specific in sphingomyelin-containing membranes. They are structurally similar to members of the fungal fruit-body lectin family that bind cell-surface exposed Thomsen–Friedenreich antigen (Tf antigen). Agaricus bisporus lectin is a tetramer and each monomer presents a novel fold with two beta sheets connected by a helix-loop-helix motif. The T-antigen disaccharide, Galβ1,3GalNAc, a mediator of the antiproliferative effects of the protein, binds at a shallow depression on the surface of the molecule. The binding of N-acetylgalactosamine overlaps with that moiety of the T antigen but, surprisingly, N-acetylglucosamine, which differs from N-acetylgalactosamine only in the configuration of epimeric hydroxyl 4, binds at a totally different site on the opposite side of the helix-loop-helix motif. Thus, this lectin has two distinct binding sites per monomer that recognize the different configuration of a single epimeric hydroxyl.5
- 3.
Family resembling β-trefoil domain lectin. This type of lectin has a barbell-like structure lacking alpha-helices or beta-sheets where individual lobes contain subdomains able to bind oligosaccharide. Lectins from Clitocybe nebularis, Coprino psiscinerea, Laetiporus sulphureus, Marasmius oreades, Polyporus squamosus, and Sclerotinia sclerotiorum belong to this family. The recombinant lectin of C. nebularis (rCNL) agglutinates human blood group A erythrocytes and is specific for the unique glycan N,N′-diacetyllactosediamine (GalNAcβ1,4GlcNAc, LacdiNAc), as demonstrated by glycan microarray analysis (Table 1). The crystal structures of rCNL together with lactose and LacdiNAc, define its interactions with the sugars. CNL is a homodimeric lectin, each of whose monomers consists of a single ricin B lectin domain with its β-trefoil fold and one carbohydrate-binding site.22
- 4.
Family resembling galectin. The galectin family of naturally occurring galactoside-binding lectins are expressed intracellularly and extracellularly by many cell types.16 Galectins regulate many critical cell processes, including differentiation, maturation, activation, migration, and apoptosis.11 Lectins from Agrocybe cylindracea and C. psiscinerea, have been isolated. Galectin from the edible fungus A. cylindracea (ACG) has a strong preference for N-acetylneuraminyl lactose (NeuAcα2,3-lactose). The structure shows that ACG is a “proto”-type galectin composed of a β sandwich of two antiparallel sheets, each with six strands, in contrast to the five and six strands in animal galectins.2
- 5.
Fungal immunomodulatory protein family. Flammulina velutipes (Fve) lectin is a member of this family.20 The lectin of this mushroom possesses immunomodulatory activity, stimulates lymphocyte mitogenesis, suppresses systemic anaphylaxis reactions and oedema, enhances transcription of IL-2, IFN-γ and tumour necrosis factor-α (TNF-α), and agglutinates red blood cells. It appears to be a lectin with specificity for cell-surface carbohydrates complex. Fve is a non-covalently linked homodimer containing Cys, His or Met residues. It shares sequence similarity only with other fungal immunomodulatory proteins (FIPs), all of unknown structure. Fve structure suggests that dimerization, critical for the activity of FIPs, occurs by 3-D domain swapping of the N-terminal helices and is stabilized predominantly by hydrophobic interactions. The structure of Fve was the first reported in this lectin family and the first of an FNIII domain-containing protein of fungal origin.20
Aspergillus fumigatus is responsible for severe infections in immunodeficient patients, causing invasive aspergillosis. The lectin present in A. fumigatus surface is of great importance for successful fixation of conidia on human epithelia. The lectin has a molecular weight of 32kDa and is specific for terminal sialic acid residues of the carbohydrate chains.29
Aspergillus oryzae is an important fermentation starter organism in the manufacture of soybean products, such as sauce and paste. However, allergic bronchopulmonary aspergillosis caused by A. oryzae has been reported in persons living near fermentation factories. A. oryzae lectin has a strong preference for the α1,6-fucosylated sugar chain among α1,2-, α1,3-, α1,4-, and α1,6-fucosylated sugar chains.18 α-L fucopyranosyl residues are widely distributed in cell surface sugar chains and often play important roles in biological phenomena. These residues constitute a part of important antigens, such as the blood group antigen H, Lewisa and Lewisb, adhesion factor-ligands such as Lewisx, and stage-specific embryonic antigens. Increased levels of fucosyl residues and changes in fucosylation patterns, as a result of different expression levels of various fucosyltransferases, act as specific markers for developmental antigens, particularly in inflammatory processes and in various cancers.18 Recently, the presence of new lectins from Aspergillus species has been reported.25,26 However, these lectins need further molecular characterization.
Genus HistoplasmaHistoplasmosis is a disease caused by the dimorphic fungus Histoplasma capsulatum. Lung infection can occur after inhalation of airborne mycelial-phase infective propagules, which are found in special environments associated with accumulated bird or bat droppings. H. capsulatum is an intracellular pathogen that causes respiratory and systemic diseases by proliferating within phagocytic cells. The binding of H. capsulatum to murine macrophages and human erythrocytes may be mediated by the pathogen's cell wall lectins.7,27 The presence of a lectin-like component in H. capsulatum has been reported,7 revealing involvement of galactosylated surface molecules of macrophages and erythrocytes as specific-sugar residues that could be recognized by the fungal lectin.7,27
Genus CandidaThe yeast Candida glabrata represents the second major cause of clinical candidiasis in the world. The ability of this opportunistic pathogen to adhere to human epithelial and endothelial cells relies on epithelial adhesins, a large set of cell-wall proteins whose N-terminal domains are endowed with a calcium-dependent lectin activity. This feature allows the yeast cells to adhere to host cells by establishing multiple interactions with the glycans expressed on their cell membrane. The ligand-binding domain of the epithelial 1p adhesin was crystallized in complex with lactose. The protein has a β-sandwich core and a calcium-binding motif.15 Recently, a detailed structural and functional characterization of the epithelial adhesins family has been determined.17
Role of glycans in host–pathogen interactionAll fungi have cell walls which are critical to maintain cell shape and integrity in environments that range from the surface of grapes to human tissues. Cell walls are highly cross-linked structures that adapt to growth conditions in a dynamic and flexible way. The cell-wall polysaccharides that have been described so far are polymers composed by mannose, glucose, galactose, N-acetylglucosamine, and/or rhamnose, and these include mannans, glucans, chitin, galactomannans, glucomannans, rhamnomannans, and phosphomannans.6 In mannans, the mannose residues of the polymeric backbone are α-linked (usually α1,6), whereas in glucans the glucose residues are β-linked (mostly β1,3, although some are β1,6). The interconnected polymers may have other sugars attached to these backbone structures and additional modifications that are specific to each organism1. Fungal cell walls also contain covalently and non-covalently linked glycoproteins that bear N- and O-glycosidically linked glycans of myriad structures; some of these glycoproteins begin as glycosylphosphatidylinositol (GPI)-anchored proteins.6
The Candida cell wall consists of mannans similarly to those in Saccharomyces cerevisiae, but they are termed phosphopeptidomannans. It also produces short β1,2-linked mannose glycans that are highly antigenic. These unusual β1,2-linked mannose glycans are also expressed on phospholipomannan antigens (PLMs).5 PLMs contain phytoceramide derivatives of myo-inositol phosphate to which mannose and long polysaccharides of β1,2-linked mannose are bound. The cell wall also contains β1,3- and β1,6-linked glucans and chitin.6 The O-glycosidically linked glycans of Candida albicans are short mannose-containing chains that have α1,2-linked mannose, but lack the α1,3-linked mannose caps found in S. cerevisiae. C. albicans mannans are also important in their interactions with host cells, including macrophages and dendritic cells.6 In particular, these structures are recognized by the mannose receptor and by Dectin-2. These are C-type lectins expressed by immune cells and they are important in both innate and adaptive immune responses. PLMs may be shed by C. albicans and, through interactions with Toll receptors (TLR-2), they can induce NF-κB activation and cytokine responses such as TNF-α secretion. Galectin-3, a ubiquitous member of the galectin family of lectins that is highly expressed in macrophages, also appears to recognize C. albicans expressing β1,2-linked mannose residues, resulting in the opsonization of the yeast.6
Cryptococcus neoformans is a common soil-dwelling, encapsulated fungus. It latently infects healthy people but causes severe disease in immunocompromised individuals which is an AIDS-defining illness.6C. neoformans is unique among pathogenic fungi in having an extensive polysaccharide capsule that is required for its virulence. Two major capsular polysaccharides, named for their monosaccharide components, are glucuronoxylomannan (GXM) and a galactoxylomannan (GalXM). GXM is an extended α1,3 mannan substituted with β1,2-Xyl, β1,4-Xyl, and β1,2-GlcA, and a subset of the mannose residues is 6-O-acetylated. Several serotypes of C. neoformans differ in the xylose modifications of the repeating unit. The second polymer, GalXM, is based on α1,6-galactan, with side chains of galactose, mannose, and xylose.6 The capsule is a dynamic structure that can change in thickness and composition depending on the environment and growth conditions. Under standard in vitro conditions, the capsule is approximately 1–2μm in diameter, but it can be much thicker, especially in the context of mammalian infection. Association of the capsule with the cell surface relies on a cell-wall component, α1,3-glucan. Although α1,3-glucan is not present in the cell walls of S. cerevisiae or C. albicans, it is common in other fungi.6
Glycans on fungi pathogens are expressed in multi- and polyvalent arrays that bind host lectins and initiate effector functions such as cross-linking and/or stimulation of an adaptive immune response. The glycan epitopes expressed by foreign pathogens are often expressed by the host, which raises the question of the molecular basis of pattern recognition of these epitopes by host lectins. Several classes of host lectins, which include calcium-dependent (C-Type) lectins, sialic acid-binding immunoglobulin-like lectin (siglecs), and galectins are currently defined as pattern recognition receptors. Finally, in the host–pathogen interaction, lectins and glycans from fungi and lectins and glycans from the host are important to avoid disease.
Conflict of interestThe authors declare no conflicts of interest.