Two-phase olive-mill wastes (or “alperujo”) exhibit highly phytotoxic properties, mainly due to phenols. A valuable option for alperujo is its agricultural use, provided that no phytotoxic effects occur.
AimsThe present investigation was aimed at evaluating the efficacy of two strains of the lignin-degrading fungus Flammulina velutipes to colonize alperujo in order to produce edible mushrooms and to achieve its detoxification.
MethodsSome important cultural characters related to mushroom production (earliness, biological efficiency and quality of basidiomes) were estimated. The production of lignocellulolytic enzymes, phenol removal and detoxification of the substrate was evaluated.
ResultsHigh biological efficiencies (70.8%) were obtained at 12°C with F. velutipes strain BAFC 670/06 in a substrate containing poplar wood shavings and 90% of alperujo. The nature of the substrate did not seem to exert an important influence on pileus and stem morphology; nevertheless shortest stems were observed at higher temperatures. Endo-β-1,4-glucanase, endo-β-1,4-xylanase, laccase and Mn-peroxidase activities were detected in the extracts recovered from the solid-state cultures. Both F. velutipes strains were effective in removing the phenolic compounds. The initial concentration in the substrate with 90% alperujo was reduced in the case of F. velutipes BAFC 1763 by 84.31%, and 40.15% by F. velutipes BAFC 670/06. Germinability experiments on Raphanus sativus, showed that alperujo phytotoxicity was significantly reduced by F. velutipes cultures.
ConclusionsThe experimented changes by the spent mushroom substrate resulting from F. velutipes cultivation with high amount of alperujo would allow its reuse for agricultural purposes.
El alperujo, subproducto de las almazaras durante la extracción del aceite de oliva, tiene propiedades fitotóxicas importantes debido a su alto contenido fenólico. Su utilización en la agricultura es una opción válida, pero deben eliminarse sus efectos fitotóxicos.
ObjetivosEl objetivo de este trabajo fue evaluar la eficacia de dos cepas del hongo ligninolítico Flammulina velutipes de crecer en sustratos con alto contenido de alperujo, detoxificarlo y producir basidiomas comestibles.
MétodosSe estudiaron las principales características relacionadas con el cultivo para la producción de basidiomas: tiempo de aparición de primordios, eficiencia biológica y calidad. Se evaluó la producción de enzimas lignocelulolíticas, la remoción de compuestos fenólicos y la detoxificación del sustrato.
ResultadosSe obtuvieron altos valores de eficiencia biológica (70,8%) a 12°C con la cepa BAFC 670/06 de F. velutipes en un sustrato compuesto de viruta de álamo y 90% de alperujo. La naturaleza del sustrato al parecer no ejerció influencias importantes en la morfología de los basidiomas, aunque a altas temperaturas los estípites presentaron una menor longitud. Se detectó actividad endo-β-1,4-glucanasa, endo-β-1,4-xilanasa, lacasa y Mn-peroxidasa en extractos recuperados de cultivos en estado sólido. Ambas cepas fueron efectivas en la reducción del contenido de fenoles del sustrato, reducción que alcanzó el 84,31% con F. velutipes BAFC 1763 y el 40,15% con F. velutipes BAFC 670/06. Los ensayos de germinación de semillas de Raphanus sativus mostraron una significativa reducción de la fitotoxicidad del alperujo.
ConclusionesLos cambios experimentados por el sustrato remanente del cultivo de F. velutipes con altas concentraciones de alperujo podrían permitir su reutilización con fines agrícolas.
The white-rot fungus Flammulina velutipes is a xylophagous fungus belonging to the Physalacriaceae family, with health and medicinal benefits, widely cultivated in East Asia.37F. velutipes is one of the six most cultivated mushroom species in the world; over 300,000t of this mushroom are produced per year.28 It is particularly known for its taste, and preventive as well as curative properties for liver diseases and gastro enteric ulcers. In addition, it has also been reported to contain immunomodulatory, antitumor and antibiotic substances.37 Its distribution is limited to temperate zones of the world because a cold period is required for fruiting.37 Its genome has been recently sequenced and reveals a high capacity for lignocellulose degradation.25 Moreover F. velutipes strain Fv-1 demonstrated its potential for the conversion of lignocellulosic biomass to ethanol by consolidated bioprocessing, a methodology that combines enzyme production, enzymatic saccharification and ethanol fermentation in one step, thus reducing the total cost of bioethanol production.16
Since F. velutipes grows on wood in nature, mixtures of lignocellulosic materials have been utilized as substrates in the commercial production of the mushroom. A common substrate used for its production is sawdust with agricultural residues, such as corncobs, cottonseed husk, sugarcane bagasse, etc.37
The olive oil extraction industry produces high amounts of olive wastes. One emerging technology for olive-oil extraction consists of a continuous centrifugation two-phase process that generates a liquid phase (olive-oil) and a semi-solid organic waste (alperujo), which are then dried and extracted with solvents to obtain an extra yield of oil and a dry olive mill residue (DOR).34 Alperujo is a fibrous lignocellulosic paste which has a water content of about 65%, a slightly acidic pH and a very high content of organic matter (90.66%), mainly composed of lignin, hemicellulose and cellulose (38.82%, 29.70% and 23.47%, w/w, of total organic matter, respectively). It has also a considerable proportion of N [TN (g·kg−1) 11.99] and phenolic compounds (1.36%, w/w, of total organic matter).21
To preserve the ecology of olive oil producer's countries, degradation of these wastes have been studied using thermal, physico-chemical processes and biological treatments.21 Several studies evaluated its detoxification through solid state fermentation and the addition of appropriate co-composting materials to deal with the particular structure of this effluent, using aerobic or anaerobic microorganisms. Apart from the exploitation of mixed microbial communities present throughout the composting process, the inoculation with white-rot basidiomycetes has also been used to decrease the contents of phenolics and phytotoxicity of the alperujo.2,13,34 White-rot fungi are able to secrete specific ligninolytic enzymes causing significant phenolic removal.31 Moreover, if edible mushrooms were utilized, a double goal could be achieved, decreasing the toxicity of this waste by means of the ligninolytic enzymes secreted by the fungus, while producing appreciated basidiomes.42
Up to now, the use of by-products from the olive-oil industry for mushrooms production has been scarcely investigated.43 Olive-mill wastewater and olive-press cake were used as substrates for Pleurotus cultivation,10,42 olive-mill by-products were used for Agaricus bisporus commercial exploitation,1 and alperujo composted with wheat straw was evaluated as substrate for basidiome production of the edible mushrooms Agrocybe cylindracea, Pleurotus cystidiosus, Pleurotus eryngii, Pleurotus ostreatus and Pleurotus pulmonarius.30,43
The aims of this work were: (i) to study the capacity of two F. velutipes strains to grow with different alperujo concentrations and temperatures while producing lignocellulolytic enzymes, (ii) to evaluate if alperujo concentration influences yield values and morphological properties of the basidiomes obtained, and (iii) to assess the ability of F. velutipes for phenol removal and detoxification in this substrate.
Materials and methodsFungal strains and culture conditionsF. velutipes strain BAFC 1763 (Fungal Collection of the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires) and BAFC 670/06 (IIB-INTECH Collection of Fungal Cultures) were used. Both strains were maintained in Petri dishes at 4°C on ME agar (malt extract 1.2%, glucose 1%, and agar 2%).
Spawn productionSpawn production was prepared in 350ml glass jars filled with boiled wheat grains (Triticum durum) and 1% (w/w) calcium carbonate (CaCO3). Jars were sterilized for 1.5h at 121°C, cooled and inoculated with a 1cm diameter plug of mycelium grown on Nobles’ medium,23 and then they were incubated at 25°C, in the dark, with periodical shaking. Time required for spawn production was recorded.
Substrate preparationPolypropylene bags of 20cm×40cm were filled with 100g (dry weight) of substrate [Populus wood shavings, alperujo (0%, 30%, 60% or 90%, w/w), and 3% CaCO3]. Humidity was adjusted (w/w) to 75%. Bags were stopped with cotton plugs held by PVC (polyvinyl chloride) cylinders and autoclaved at 121°C for 2h. After cooling, bags were inoculated with 5% (wet weight) of spawn, and were incubated at 25°C in the dark until total substrate colonization (21–25 days).
Basidiomes inductionAfter total colonization by the mycelium, polypropylene bags with the upper surface opened (with a neck of 10cm approx. created from the same bag) were transferred to the basidiome production rooms. Cropping conditions to induce basidiome formation were 12h light/12h dark photoperiod (2000lx of fluorescent light), 75–85% humidity levels and watering by automatic spray provided for 2min, 3 times every day. Three different temperatures were assayed for basidiome production: 6±1°C, 12±1°C and 22±1°C.
Cropping period, crop yield and morphological traits assessmentUp to three flushes were harvested during the cropping period (80 days between the induction day and the last harvest) in the different substrates. Mature basidiomes (80% of expanded pileus) were collected manually. The parameters to test the suitability of the substrates under study for the cultivation of F. velutipes were (a) earliness, defined as the time elapsed between the day of inoculation and the day of primordial formation, (b) biological efficiency (BE), calculated as the percentage ratio of fresh mushrooms weight over the dry weight of the substrate, and (c) morphological quality traits: (i) pileus width and length and (ii) stem length and diameter.
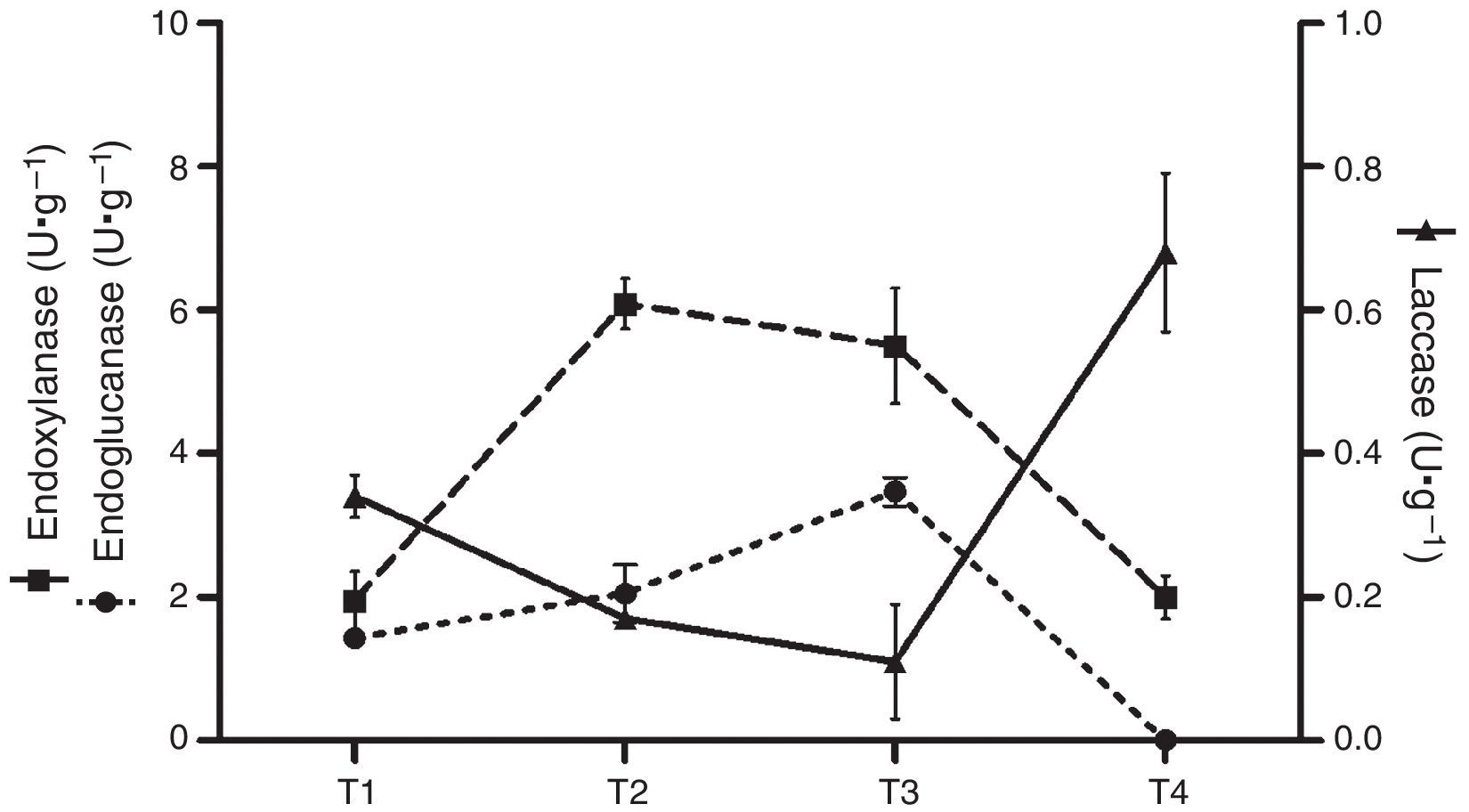
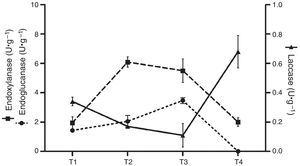
Analytical determinationsEnzyme activitiesLaccase activity (E.C.:1.10.3.2) was measured using 2,6-dimetoxiphenol (DMP) 5mM in 0.1M sodium acetate buffer (pH 3.6) at 50°C. Oxidation of DMP was determined by the increase in A469 (¿469=27mM·cm−1).26 Manganese peroxidase activity (MnP) (E.C.:1.11.1.13) was measured using phenol red as the substrate in 0.1M sodium dimethyl succinate buffer (pH 4.5) (¿610=22mM·cm−1) at 50°C.6 Endo-β-1,4-glucanase (E.C.:3.2.1.4) and endo-β-1,4-xylanase (E.C.:3.2.1.8) activities were determined by measuring the reducing sugars released from carboxymethylcellulose or oat xylan, respectively, as substrates, in 50mM sodium acetate buffer, pH 4.8. Liberated reducing sugars were quantified by the Somogyi–Nelson method,22 using either glucose or xylose as standards.40 Cellulolytic and xylanolytic activities were determined at 50°C. Enzyme activity was expressed in International Units (U) as the amount of enzyme required to release 1μmol of product in 1min. In terms of production, the activity was defined as U·g−1 dry residue (substrate plus mycelium). Samples of substrates colonized by mycelium were collected at different stages of the solid-state fermentation: T1, after complete colonization of the substrate; T2, after the end of first flush; T3, after the end of the second flush (if present); T4, spent substrate after the last mushroom harvest. Crude extracts were obtained by adding distilled water to the samples from each freshly harvested culture (5:1, w/w), stirring for 20min, followed by filtration and centrifugation. The supernatants were stored at 20°C until needed.
Total phenolic compounds quantificationDried solid samples of non-inoculated and spent substrates were extracted with methanol:water 80:20 (v/v) (5:1, w/w), stirring for 2h at 120rpm, followed by filtration and centrifugation. The quantification of the total phenolic compounds was based on the Folin–Ciocalteu reaction, according to the method of Singleton et al.,38 measuring the absorbance at 765nm. Gallic acid was used as standard for the quantification.
Phytotoxicity bioassayThe phytotoxicity of the extract obtained from Flammulina's spent mushroom was determined according to the method described by Zucconi et al.44 using Raphanus sativus seeds incubated for 48h at 28°C with either (a) control:water; (b) the extract obtained from the non-inoculated substrate and (c) the extract obtained from the spent substrate after the last mushroom harvest. The germination index (GI) was calculated according to the expression: GI=[(G/Go)/(L/Lo)]×100, where Go and Lo are, respectively, the germination percentage and radicle growth of the control. Crude extracts were obtained by adding distilled water to the samples from each freshly harvested culture (5:1, w/w), stirring for 20min, and followed by filtration and centrifugation.
Experimental design and statistical treatmentsFor basidiome production each treatment consisted of ten replicates. Data of analytical determinations are the average of the results of at least three replications with a standard error of less than 5%. Analysis of variance (ANOVA) and repeated measures analysis were tested by the software Statistica v.7.0. The assumptions of normality and homogeneity of variances were checked by means of Kolmogorov–Smirnov and Bartlett tests, respectively, for the validity of ANOVA method. The significant differences between treatments were compared by Tukey's test at 5% level of probability.
ResultsEffect of alperujo addition and temperature of incubation on F. velutipes basidiome productionBoth strains of F. velutipes took 21–25 days to complete the colonization of all the substrates assayed. Growth was assessed visually, and while a dense mycelial mat could be observed in the substrates supplemented with alperujo, only scatter hyphae were seen on the control with solely poplar wood shavings. Lignocellulosic materials are generally low in protein content, insufficient for mushroom cultivation. Since the C:N ratio plays an important role in spawn running and the growth of basidiomes, nitrogen supplementation is an important factor for the growth and yield of mushrooms. The high content of organic matter provided by the alperujo might support better mycelial growth.
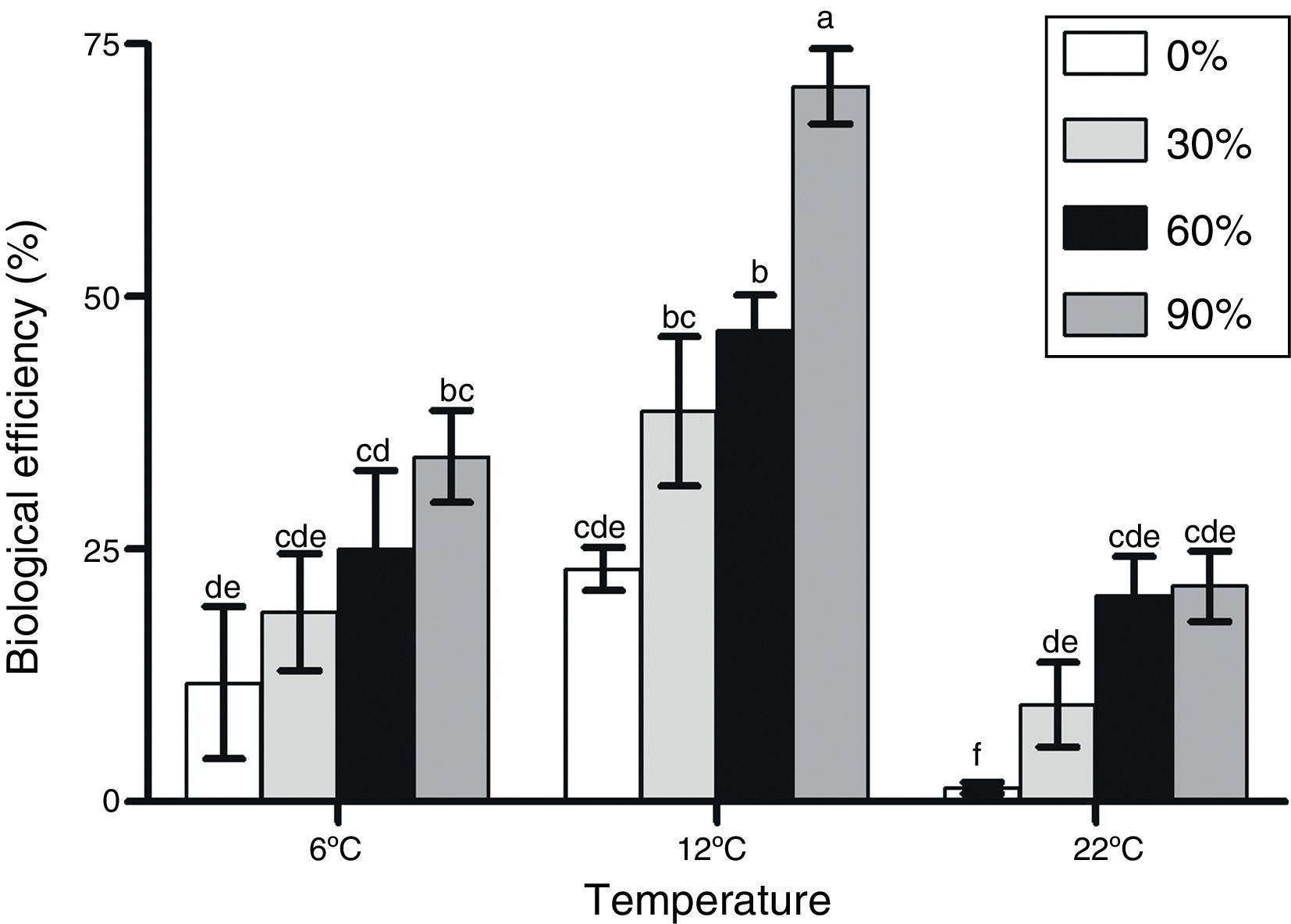
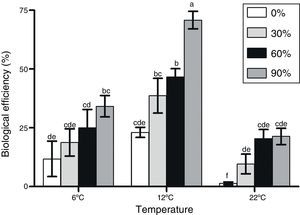
F. velutipes strain BAFC 670/06 demonstrated an exceptionally good adaptation to olive mill waste containing substrates since most of the alperujo media provided significantly higher BEs values in comparison to the control. Moreover, with alperujo addition mushroom primordia were formed within a shorter time period after substrate inoculation; the first flush was observed between 13 and 33 days after initial induction (Table 1). The highest BEs were obtained at 12°C with F. velutipes strain BAFC 670/06, and BEs improved with increasing alperujo concentrations up to a maximum of 70.8 with 90% of this olive waste (Fig. 1). No significant differences were observed in BEs obtained with substrates containing 60 and 30% alperujo (46.60% and 38.68%, respectively) at 12°C. More than 60, 75 and 90% of the total production was cropped during the first flush with 90%, 60% and 30% alperujo content, respectively, at 12°C. Lower BEs were attained at 6°C and 22°C: at 6°C BEs varied from 11.8 to 34.17%, while at 22°C BEs ranged from 1.4 up to 21.33% with different amounts of alperujo; the highest values were obtained with 90% (Fig. 1).
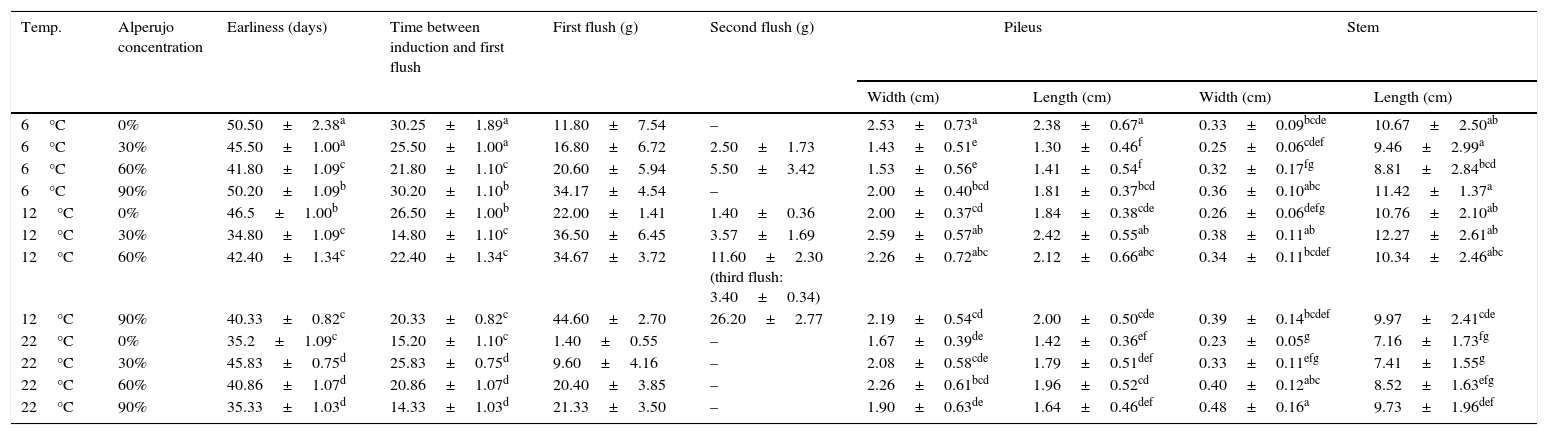
Effect of alperujo addition and temperature of induction on F. velutipes strain 670/06 basidiome production.
| Temp. | Alperujo concentration | Earliness (days) | Time between induction and first flush | First flush (g) | Second flush (g) | Pileus | Stem | ||
|---|---|---|---|---|---|---|---|---|---|
| Width (cm) | Length (cm) | Width (cm) | Length (cm) | ||||||
| 6°C | 0% | 50.50±2.38a | 30.25±1.89a | 11.80±7.54 | – | 2.53±0.73a | 2.38±0.67a | 0.33±0.09bcde | 10.67±2.50ab |
| 6°C | 30% | 45.50±1.00a | 25.50±1.00a | 16.80±6.72 | 2.50±1.73 | 1.43±0.51e | 1.30±0.46f | 0.25±0.06cdef | 9.46±2.99a |
| 6°C | 60% | 41.80±1.09c | 21.80±1.10c | 20.60±5.94 | 5.50±3.42 | 1.53±0.56e | 1.41±0.54f | 0.32±0.17fg | 8.81±2.84bcd |
| 6°C | 90% | 50.20±1.09b | 30.20±1.10b | 34.17±4.54 | – | 2.00±0.40bcd | 1.81±0.37bcd | 0.36±0.10abc | 11.42±1.37a |
| 12°C | 0% | 46.5±1.00b | 26.50±1.00b | 22.00±1.41 | 1.40±0.36 | 2.00±0.37cd | 1.84±0.38cde | 0.26±0.06defg | 10.76±2.10ab |
| 12°C | 30% | 34.80±1.09c | 14.80±1.10c | 36.50±6.45 | 3.57±1.69 | 2.59±0.57ab | 2.42±0.55ab | 0.38±0.11ab | 12.27±2.61ab |
| 12°C | 60% | 42.40±1.34c | 22.40±1.34c | 34.67±3.72 | 11.60±2.30 (third flush: 3.40±0.34) | 2.26±0.72abc | 2.12±0.66abc | 0.34±0.11bcdef | 10.34±2.46abc |
| 12°C | 90% | 40.33±0.82c | 20.33±0.82c | 44.60±2.70 | 26.20±2.77 | 2.19±0.54cd | 2.00±0.50cde | 0.39±0.14bcdef | 9.97±2.41cde |
| 22°C | 0% | 35.2±1.09c | 15.20±1.10c | 1.40±0.55 | – | 1.67±0.39de | 1.42±0.36ef | 0.23±0.05g | 7.16±1.73fg |
| 22°C | 30% | 45.83±0.75d | 25.83±0.75d | 9.60±4.16 | – | 2.08±0.58cde | 1.79±0.51def | 0.33±0.11efg | 7.41±1.55g |
| 22°C | 60% | 40.86±1.07d | 20.86±1.07d | 20.40±3.85 | – | 2.26±0.61bcd | 1.96±0.52cd | 0.40±0.12abc | 8.52±1.63efg |
| 22°C | 90% | 35.33±1.03d | 14.33±1.03d | 21.33±3.50 | – | 1.90±0.63de | 1.64±0.46def | 0.48±0.16a | 9.73±1.96def |
Data are the mean±standard deviation of three determinations. Means followed by the same letter are not significantly different according to Tukey's test.
The lowest BEs gotten at 22°C might be related with various aborted primordia observed. Strain 1763 only produced basidiomes on 60% of alperujo at 12°C and 22°C, and the BEs were less than 10% (data not shown).
Pileus width and length, and stem length and diameter, were not significantly different among most of the treatments for the same strain, and hence the nature of the substrate did not seem to exert any important influence (exception i.e. in stem morphology with 90% of alperujo). On the contrary, the temperature of incubation held sway over morphology of F. velutipes basidiomes, resulting in mushrooms with shorter stems at 22°C (Table 1).
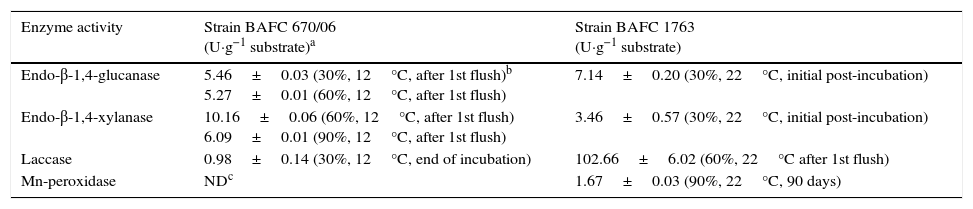
Enzyme activitiesCellulolytic, xylanolytic and ligninolytic activities were detected in the extracts recovered from the solid-state cultures. Table 2 summarizes the highest titers of the different enzyme activities attained by both strains (and the day and medium in which these values were achieved). Time course of enzyme production in the conditions that rendered the highest BE, F. velutipes strain BAFC 670/06 grown at 12°C with 90% of alperujo, is depicted in Fig. 2. Hydrolytic activities showed their highest values at initial stages of cultivation: endo-β-1,4-glucanase utmost titers detected were 5.46±0.03U·g−1 (30%, 12°C) and 5.27±0.01U·g−1 (60%, 12°C) for 670/06 strain, and 7.14±0.20 (0%, 22°C, and 30%, 22°C) for 1763 strain, while endo-β-1,4-xylanase peaks were 10.16±0.06U·g−1 (60%, 12°C) and 6.09±0.01U·g−1 (90%, 12°C) for 670/06 strain. On the contrary, laccase activity showed its highest activity at final stages, at second flush or at the end of the culture period (80 days). F. velutipes BAFC 1763 displayed a higher activity of laccase [102.66±6.02U·g−1 (60%, 22°C)], and MnP activity was only detected in this strain (1.67±0.03U·g−1 with 90% alperujo at 22°C). Highest laccase activity detected in strain BAFC 670/06 was 0.98±0.14U·g−1 with 30% alperujo at 12°C at the end of incubation. Laccase activity was influenced by the concentration of alperujo added to the substrate. Highest laccase activities recorded for strain BAFC 1763 at 12°C with 0, 30, 60 and 90% alperujo were respectively: 0.64±0.03; 5.46±0.23; 21.74±1.05 and 27.11±2.90U·g−1. Strain BAFC 670/06 produced the highest laccase activity with 30% alperujo (0.98±0.14U·g−1 at 12°C); laccase activity was 0.68±0.11U·g−1 with 90% alperujo at the same temperature while it not exceeded 0.35±0.07U·g−1 in the control without alperujo in the same conditions.
Extracellular lignocellulolytic activities displayed by F. velutipes strains on poplar wood shavings with different alperujo concentrations.
| Enzyme activity | Strain BAFC 670/06 (U·g−1 substrate)a | Strain BAFC 1763 (U·g−1 substrate) |
|---|---|---|
| Endo-β-1,4-glucanase | 5.46±0.03 (30%, 12°C, after 1st flush)b 5.27±0.01 (60%, 12°C, after 1st flush) | 7.14±0.20 (30%, 22°C, initial post-incubation) |
| Endo-β-1,4-xylanase | 10.16±0.06 (60%, 12°C, after 1st flush) 6.09±0.01 (90%, 12°C, after 1st flush) | 3.46±0.57 (30%, 22°C, initial post-incubation) |
| Laccase | 0.98±0.14 (30%, 12°C, end of incubation) | 102.66±6.02 (60%, 22°C after 1st flush) |
| Mn-peroxidase | NDc | 1.67±0.03 (90%, 22°C, 90 days) |
Both F. velutipes strains were effective in removing the phenolic compounds. Initial phenolic concentration in substrates with 30%, 60 and 90% of alperujo were respectively 2.61, 3.14 and 3.76mg·g−1 (expressed as gallic acid equivalents). Phenol concentration at the end of the incubation in the substrate with 90% alperujo was reduced by 84.31% at 12°C by F. velutipes BAFC 1763, and 42.28% by F. velutipes BAFC 670/06. Phenol removal by F. velutipes BAFC 1763 reached values of 93.06% and 87.26% with 30% and 60% of alperujo content, while those attained by F. velutipes BAFC 670/06 with 30% and 60% of alperujo at 12°C were respectively 33.71% and 46.49%. GI of the treated substrate (with 90% of alperujo) was similar, or even higher than the control value (100%): 127% and 97% for strains BAFC 670/06 and 1763, respectively. GI value in the non-treated substrate was 26.67%.
DiscussionThis work evaluated for the first time the potential use of olive mill wastes as substrate for F. velutipes cultivation. F. velutipes strain BAFC 670/06 formed primordia in a shorter time on substrates with alperujo. On the contrary, in previous works, always olive waste amendment had a distinct adverse effect on earliness.30,42,43 Taking into account the nature of the residue used to cultivate the mushroom, BEs obtained in this work are acceptable and comparable to those obtained in other publications when growing F. velutipes using different formulation substrates: i.e. 50.9% using paddy straw with additives,14 90–106% on paddy straw,39 73% with maize straw,7 56% on coffee husks12 and 78% while using coffee spent grounds.12
Pileus width and length, and stem length and diameter, were not significantly different among most of the treatments for the same strain when varying the amount of alperujo. Likewise, in the cases of P. ostreatus and P. pulmonarius no significant differences were observed in the size of the mushrooms obtained when assaying substrates with different alperujo concentrations.43 Seven oyster mushroom strains were cultivated in wheat straw bags supplemented with up to 90% alperujo, and most of them showed no significant differences on cultivation parameters and basidiomes quality (except for color) between the control and the substrates supplemented with up to 50% alperujo, although high alperujo concentrations resulted in a significant yield, biological efficiency and productivity decrease, retarding of pinning and flushing.30 Alperujo additions affected the yield, enzyme secretion, color and texture of the Pleurotus specimens obtained.27 In both strains of F. velutipes assayed in this work, alperujo addition did not cause a decrease in mushroom size.
In order to obtain the nutrients required for growth and fruiting, F. velutipes is assumed to secrete the hydrolytic/oxidative enzymes that catalyze the degradation of the major macromolecular components (cellulose, hemicellulose and lignin) of its growth substrate. However, compared with other cultivated mushrooms, very little is known about the nature of the lignocellulolytic enzymes produced by F. velutipes, the parameters affecting their production, and enzyme activity profiles during different stages of the developmental cycle.17,36 The production of these enzymes is important in substrate colonization, as well as decisive in basidiome production.24 Moreover the production of ligninolytic enzymes by the fungus might be involved in its capacity to grow and detoxify substrates with high contents of phenolic compounds like alperujo.
Hydrolysis of cellulose and hemicellulose provides the nutrients required for vegetative growth as a prelude to basidiome production. Endoglucanase and endoxylanase activities increased with the incubation time and also during the formation of basidiomes for both strains. Matsumoto19 also found that cellulase and xylanase activities increased during the development of Lentinus edodes basidiomes cultivated on eucalyptus sawdust, with highest levels during mushroom maturation. Luz et al.15 registered similar results when incubating P. ostreatus in different agro industrial wastes. Kurt and Buyukalaca9 detected the highest endoglucanase activities when incubating P. ostreatus and Pleurotus sajor-caju in different agro industrial wastes after first flush and on the 5th day of mycelial growth. The increase in the enzyme activities during basidiome production may be due to the fungus’ need to mobilize large amounts of carbon for mushroom formation.18
High ligninolytic activities during growth phases in lignocellulosic substrates, with drastic reductions during the period of basidiome formation, were observed in the white-rotting basidiomycetes P. ostreatus,9P. sajor-caju,9Lentinus tigrinus,11L. edodes,18,24A. bisporus41 and Grifola frondosa.20 In contrast, during Volvariella volvacea solid state fermentation, laccase activity increased greatly after vegetative growth at basidiome initiation and stayed at high level until the basidiomes matured.4 Laccase activity was influenced by the concentration of alperujo added to the substrate. Ruiz Rodriguez et al.31 also found an increase in laccase and peroxidase levels secreted by P. ostreatus and P. pulmonarius on substrates supplemented with olive mill wastes than on control.
DOR and its components have been described as a source of phenols and stimulators of fungal growth that induce several enzymes such as MnP and Lac.5,29,35
Ko et al.8 evaluated endoglucanase, endoxylanase and laccase activities recovered from spent mushroom compost of F. velutipes grown in sawdust; specific activities obtained were respectively 151, 110 and 48.5nkat·g−1. Compared with these data, our results showed higher recovery of all the enzyme activities evaluated. Moreover, as far as we know this is the first report of MnP activity in F. velutipes.
Due to its content in organic matter and mineral nutrients, alperujo might be employed for agronomic purposes.2 However, the main technical constraint to its biological use is the presence of a relevant phenolic fraction, the concentration of which may easily range from 12 to 26g·kg−1, exhibiting significant phytotoxicity.32 Both spent mushroom substrates under trial showed low phytotoxicity (GI>60%) within the range of safety for agronomic use.44 Thus, the spent mushroom compost derived from F. velutipes cultivation might be used as organic fertilizer.1F. velutipes BAFC 1763 removed these toxic compounds more efficiently probably by synthesizing and excreting higher titers of ligninolytic enzymes (laccase and MnP) than the strain BAFC 670/06 into the alperujo. The effect of laccase and MnP on DOR, as well as their aqueous and organic extractives, has to some extent been described in vivo and in vitro.29Coriolopsis rigida, another white-rot fungus, is capable of transforming certain phytotoxic monomeric phenols in DOR, into non-phytotoxic polymeric phenols.3 The degree of polymerization of these phenols may limit the accessibility via plant cell membrane, decreasing phytotoxicity.33 The incubation of DOR with Bjerkandera adusta also produced a sharp decrease in total phenolic content in 4 weeks, as well as a reduction in phytotoxicity.29
In conclusion, the results achieved in this investigation contribute to expand the knowledge on the lignocellulolytic enzyme system of F. velutipes, scarcely investigated up till now. Taking into account that F. velutipes successfully colonized this waste, this substrate formula might be considered for its growth. The inclusion of poplar wood shavings provides nutrients and enhances gas exchange and water retention, thus facilitating the degradation of this toxic compound.13 Both F. velutipes strains decreased the phenolic content whilst at the same time yielded a less phytotoxic substrate. F. velutipes BAFC 1763 cultures in alperujo/poplar wood shavings mixtures resulted in aqueous extracts containing about 80% less phenolic compounds, which inhibited seed germination to a much lesser extent. Experiments comparing abiotic control with the fungal treated substrate containing 90% of alperujo, showed that phytotoxicity was totally suppressed in the waste that underwent 100 days of fungal treatment. The experimented changes undergone by alperujo after degradation would allow its reuse (after the harvest of the mushrooms) for agricultural purposes as organic amendments or soil conditioner for growing plants.32
Conflict of interestThe authors declare that there are no conflicts of interest.
The authors thank University of Buenos Aires and CONICET (Argentina) for the financial assistance.