Pythium insidiosum is an oomycete classified in the kingdom Stramenopila. P. insidiosum hyphae are not able to initiate infection without the secretion of hydrolytic enzymes, which are considered an important factor in microbial virulence.
AimsTo evaluate the extracellular enzymatic activity of 14 Brazilian P. insidiosum isolates and a standard strain (ATCC 58637) by the API-ZYM System screening method.
MethodsZoospores were grown in RPMI 1640 broth, and 65μL of the liquid phase were inoculated in each cupule of the API-ZYM strips.
ResultsDifferences in the enzymatic activities were observed among the isolates, although phosphohydrolases and ester hydrolases were conspicuous among all isolates. β-glucosidase was also present in most of the isolates. Enzymatic activities of α-glucosidase and chymotrypsin were not observed, differing from a previous study involving Australian isolates and intracellular enzymes.
ConclusionsThe discrepancy in the enzymatic profile observed among Brazilian P. insidiosum isolates reflects the phenotypic variations found in susceptibility tests.
Pythium insidiosum es un patógeno clasificado en el Reino Stramenopila, phylum Oomycota, clase Oomycetes, familia Pythiaceae. Sus hifas no son capaces de iniciar una infección sin la secreción de enzimas hidrolíticas, que se consideran un importante factor de virulencia microbiana.
ObjetivosExaminar la actividad enzimática extracelular de 14 aislamientos de P. insidiosum en Brasil y de una cepa de control (ATCC 58637) mediante el método de cribado API-ZYM®.
MétodosSe cultivaron las zoosporas en caldo RPMI 1640, y se inocularon 65μL de la fase líquida de las suspensiones celulares en cada cúpula de las galerías del método API-ZYM®.
ResultadosEntre los aislamientos se observaron diferencias en las actividades enzimáticas, aunque las fosfohidrolasas y éster hidrolasas fueron evidentes en todos los aislamientos. La enzima β-glucosidasa también estaba presente en la mayoría de los aislamientos. No se detectaron actividades enzimáticas de α-glucosidasa y quimotripsina, a diferencia de lo observado en un estudio previo efectuado en Australia sobre aislamientos y enzimas intracelulares.
ConclusionesLa discrepancia observada en el perfil enzimático entre aislamientos de P. insidiosum en Brasil refleja las variaciones fenotípicas identificadas en las pruebas de sensibilidad.
Among all Pythium species, P. insidiosum is the only one known to be pathogenic for mammals, with the greatest number of outbreaks occurring in horses, dogs and humans.6,9,14 This oomycetous pathogen of the kingdom Stramenopila, phylum Oomycota, develops hyphae similar to those found in true fungi. The prevailing model for infection in pythiosis suggests that the infectious propagule is an aquatic zoospore.9,10 Hyphae emitted towards the host species colonize cutaneous and subcutaneous tissues, produce intestinal lesions, invade blood vessels and can proliferate within bone.
Although the exertion of invasive pressure is crucial for tissue colonization, hyphae of Pythium species are not sufficiently powerful to penetrate solid tissues without the concerted activity of secreted enzymes.12 It is known that extracellular enzymes, proteases and phospholipases can destroy host tissue and aid fungal invasion. In the recent literature, Davis et al.4 reported substantial secreted protease activity (serine proteases) in three different P. insidiosum strains. The experiments indicated that tissue-degrading enzymes must exert a significant reduction in tissue strength to allow hyphal penetration of plant or animal tissues. As further data on the enzymatic profile of this oomycete and its intraspecific variability with regard to enzyme activity are scarce, we investigated the production of 19 hydrolytic enzymes by Brazilian P. insidiosum strains isolated from animal pythiosis.
Materials and methodsThis study included twelve Brazilian P. insidiosum strains obtained from equine pythiosis, two strains (C20, C28) re-isolated from experimentally infected rabbits (strain CBS 101555)11 and a culture collection strain (ATCC 58637). The identities of the isolates were confirmed by a PCR-based assay.1 Strains were initially cultivated in Corn Meal Agar at 37°C and subsequently subjected to zoosporogenesis.11,13 Zoospores were counted using a Neubauer chamber and 2.5 to 7.5×103 zoospores were transferred to 2mL of RPMI 1640 broth containing l-glutamine (Sigma–Aldrich, Germany) and buffered to pH 7.0 with 0.165M morpholinepropanesulfonic acid. Tubes with inocula were maintained at 37°C for 24h.
Enzymatic activities were determined by means of the API-ZYM System (bioMérieux, Marcy- l’Etoile, France) in accordance with the manufacturer's instructions. Briefly, 65μL of the liquid phases from incubated cultures were added to each well of the APY-ZYM tray and maintained at 37°C for 4h. Hyphae of P. insidiosum were carefully separated from the inoculum. The results were determined in nanomoles (nmol) of the hydrolyzed substrate according to the intensity of the colour reaction on a scale of 1–5, i.e., 1=5nmol, 2=10nmol, 3=20nmol, 4=30nmol and 5=>40nmol. The tests were carried out in triplicate and medians were submitted to Kruskal–Wallis statistic test using a significance level of 5%. Differences between the enzymatic activities of all strains and the control group (RPMI broth only) and differences among the isolates were analyzed by the Dunn's multiple comparison test.
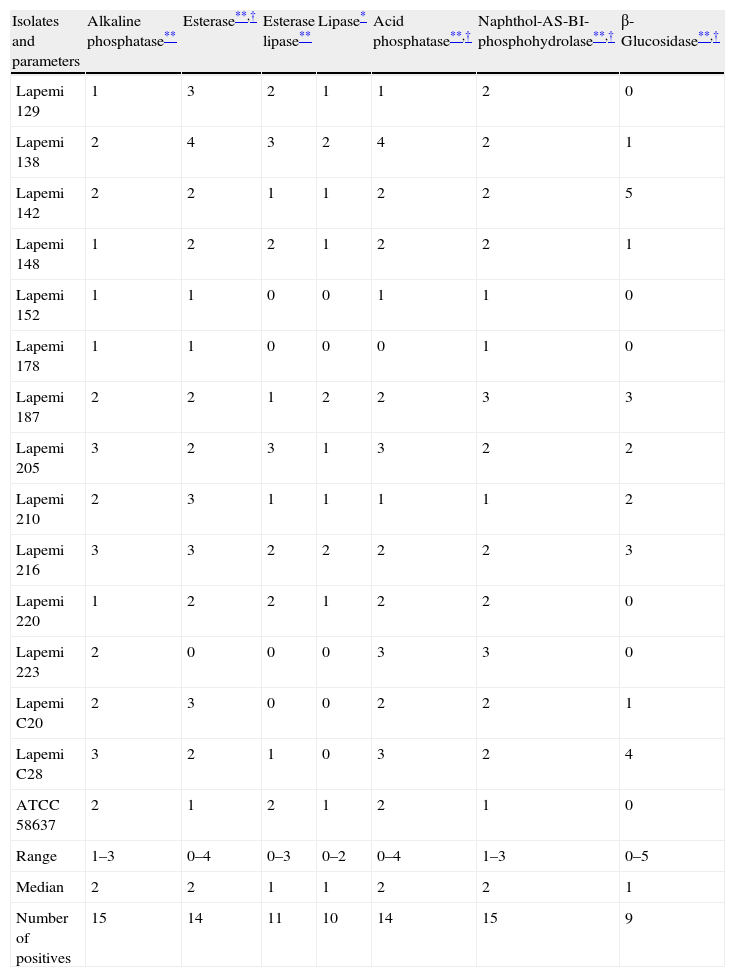
ResultsThe most conspicuous enzymatic activities shown by the 15 P. insidiosum isolates were alkaline phosphatase (ALP), esterase, esterase lipase, acid phosphatase (ACP), naphthol-AS-BI-phosphohydrolase and β-glucosidase (P<0.01). Lipase activity was also observed (P<0.05). Eight isolates also produced low levels of trypsin, but the activity of this enzyme did not differ significantly from the control (P>0.05). Within the isolates, differences in the production of β-glucosidase (P<0.001), esterase, naphthol-AS-BI-phosphohydrolase (P<0.01) and ACP (P<0.05) were observed. Data are shown in Table 1.
Median values (n=3) for the enzymatic activity of 15 Pythium insidiosum isolates obtained from horses (Lapemi 129–Lapemi 223), experimentally infected rabbits (Lapemi C20 and C28) and a culture collection strain (ATCC 58637).
| Isolates and parameters | Alkaline phosphatase** | Esterase**,† | Esterase lipase** | Lipase* | Acid phosphatase**,† | Naphthol-AS-BI-phosphohydrolase**,† | β-Glucosidase**,† |
| Lapemi 129 | 1 | 3 | 2 | 1 | 1 | 2 | 0 |
| Lapemi 138 | 2 | 4 | 3 | 2 | 4 | 2 | 1 |
| Lapemi 142 | 2 | 2 | 1 | 1 | 2 | 2 | 5 |
| Lapemi 148 | 1 | 2 | 2 | 1 | 2 | 2 | 1 |
| Lapemi 152 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Lapemi 178 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Lapemi 187 | 2 | 2 | 1 | 2 | 2 | 3 | 3 |
| Lapemi 205 | 3 | 2 | 3 | 1 | 3 | 2 | 2 |
| Lapemi 210 | 2 | 3 | 1 | 1 | 1 | 1 | 2 |
| Lapemi 216 | 3 | 3 | 2 | 2 | 2 | 2 | 3 |
| Lapemi 220 | 1 | 2 | 2 | 1 | 2 | 2 | 0 |
| Lapemi 223 | 2 | 0 | 0 | 0 | 3 | 3 | 0 |
| Lapemi C20 | 2 | 3 | 0 | 0 | 2 | 2 | 1 |
| Lapemi C28 | 3 | 2 | 1 | 0 | 3 | 2 | 4 |
| ATCC 58637 | 2 | 1 | 2 | 1 | 2 | 1 | 0 |
| Range | 1–3 | 0–4 | 0–3 | 0–2 | 0–4 | 1–3 | 0–5 |
| Median | 2 | 2 | 1 | 1 | 2 | 2 | 1 |
| Number of positives | 15 | 14 | 11 | 10 | 14 | 15 | 9 |
Our results demonstrate a high intraspecific variability in enzymatic activity. ALP and naphthol-AS-BI-phosphohydrolase activities were detected in 100% of the P. insidiosum isolates, whereas esterase and ACP were detected in 93% of the isolates. Esterase lipase, lipase and β-glucosidase activities were detected in 73%, 66% and 60% of the isolates, respectively. No differences in the enzymatic activity were observed according to the animal species from which the strain was isolated (equine or rabbit).
DiscussionThe API-ZYM system is a semiquantitative method that permits the rapid detection of 19 enzymes. Many studies have confirmed its usefulness in mycology.5,16,17 Besides the excellent growth of zoospores, the RPMI broth allowed the establishment of a standardized inoculum; we were also able to analyze the enzymes secreted at the moment that a germ tube is produced by the zoospore, as it occurs in infections.8 Moreover, bacterial contamination is easily avoided by using this broth, a rather trivial point when an enzymatic profile is required.
Differences in the enzymatic production observed among the isolates were expected. Biochemical variability or inconsistency among Brazilian P. insidiosum strains have been reported in susceptibility tests with the same isolates assayed in our study.2,11 Using western immunoblot analysis, Leal et al.8 observed important antigenic differences among Brazilian P. insidiosum field isolates. However, a more extensive database is required before a key based on phenotypic patterns can be constructed.
Shipton,15 in an effort to differentiate plant and animal isolates, found similar enzymatic profile from our study for the Australian equine derived Pythium spp., with the exception of the presence of α-glucosidase and chymotrypsin. However, instead of zoospores the author used sonicated proteins obtained from hyphal fragments grown on a glucose broth medium (35°C, 12h) for inoculating the API-ZYM tray. Based on these observations, two possible hypotheses could explain the differences in the enzymatic profiles: (1) alpha-glucosidase and chymotrypsin are specific intracellular enzymes, because only secreted enzymes were researched in our study; (2) each group studied has a specific enzymatic pattern, according to the geographic cluster in which it is included.14
Cellulases (i.e., β-glucosidase in our study) might not be particularly important in pathogenesis, since extensive cellulose degradation typically occurs only late in infection. Notwithstanding, the enzymatic action of β-glucosidase is likely to be fundamental in wall-loosening and growth due to the hydrolysis of β(1–3)-d-glucan linkages present in the P. insidiosum cell wall.3,10
The expression of phosphatases is ubiquitous among many pathogenic fungi.16,17 A highly significant positive correlation is established between the adhesion of Candida to mammalian buccal epithelial cells and ALP and ACP activity. This relationship, described for the first time by Fernanado et al.,5 implies that the phosphatases of Candida species may play a crucial role in potentiating their virulence.
The ability of the P. insidiosum zoospores to initiate an infection is due to the production of a substance that allows them to maintain tight contact with the host.9 Esterases and lipases both hydrolyze ester bonds. Whereas the lipases display high activity towards the aggregated state of its substrate, the esterases typically show highest activity towards the soluble state of its substrate.7 Shipton15 observed lipase activity in all (n=14) Australian equine-derived Pythium spp., although only one out of four plant isolates showed activity of this enzyme. Our results suggest that the expressive production of esterases and lipases by P. insidiosum aid encystment due to the reduction in surface hydrophobicity.
In conclusion, P. insidiosum zoospores grown in RPMI 1640 broth produce extracellular enzymes that diverge among the isolates. In contrast with a previous study, lack of chymotrypsin and α-glucosidase activities was observed. This might be related to genetically distinct populations of P. insidiosum strains used in both studies or to the technique employed, since only secreted enzymes were researched in our study. Further enzymatic studies on P. insidiosum isolates and non-mammalian pathogenic Pythium species, along with the biochemical characterization and purification of the enzymes observed in our study, would enhance the paper of each enzyme in the pathogenic process.
Conflict of interestsNone to declare.
This study was supported by CNPq (the National Council for Scientific and Technological Development of Brazil), Laboratório de Pesquisas Micológicas of the Universidade Federal de Santa Maria and Setor de Micologia of Faculdade de Veterinária of the Universidade Federal de Porto Alegre, RS, Brazil.