Candida albicans is the main agent that causes vulvovaginal candidiasis. Resistance among isolates to azole antifungal agents has been reported.
AimsDue to the well-known antifungal potential of curcumin, the purpose of this work was to evaluate the in vitro anticandidal activity of curcumin and its effect in the treatment of experimental vulvovaginal candidiasis.
MethodsThe anticandidal activity of curcumin was investigated against eight Candida strains by the broth microdilution assay, and its mechanism of action was evaluated by testing the binding to ergosterol. Then, the effect of curcumin in the treatment of vulvovaginal candidiasis was evaluated in an immunosuppressed, estrogen treated rat model.
ResultsCurcumin showed minimum inhibitory concentration values of 125–1000μg/ml, and the best result was observed against Candida glabrata. The compound was shown to be able to bind to the ergosterol present in the membrane, event that may be the mechanism of action. In addition, in the in vivo model of vulvovaginal candidiasis with C. albicans, treatments reduced the vaginal fungal burden in infected rats after seven days of treatment with different doses.
ConclusionsCurcumin could be considered a promising effective antifungal agent in the treatment of vulvovaginal candidiasis.
Candida albicans es la principal causante de la candidiasis vulvovaginal y algunos aislamientos pueden presentar resistencia a los antifúngicos azólicos.
ObjetivosDebido al conocido potencial antifúngico de la curcumina, el objetivo de este trabajo fue evaluar su actividad anti-Candidain vitro y su efecto en el tratamiento de la candidiasis vulvovaginal experimental.
MétodosLa actividad anti-Candida de la curcumina se evaluó frente a ocho cepas de Candida mediante un ensayo de microdilución en caldo, y su mecanismo de acción se estudió por una prueba de unión a ergosterol. Posteriormente se evaluó el efecto de la curcumina en el tratamiento de la candidiasis vulvovaginal con un modelo de rata inmunosuprimida, tratada con estrógenos.
ResultadosLa curcumina mostró valores de concentración inhibitoria mínima de 125-1.000μg/ml, y el mejor resultado se observó frente a Candida glabrata. El compuesto demostró ser capaz de unirse al ergosterol de la membrana, lo que podría ser su mecanismo de acción. Además, en el modelo in vivo de candidiasis vulvovaginal con C. albicans, los tratamientos redujeron la carga fúngica vaginal en ratas infectadas después de siete días de tratamiento con diferentes dosis.
ConclusionesLa curcumina podría considerarse un agente antifúngico eficaz prometedor en el tratamiento de la candidiasis vulvovaginal.
The most prevalent candidiasis-causing species is Candida albicans.45C. albicans is a dimorphic commensal fungus of mucosal surfaces, commonly found in the gastrointestinal and genitourinary tracts. It is the main agent that causes vulvovaginal candidiasis (VVC). The incidence of this disease is high, affecting 75% of all women at least once during their life-time. The risks associated with the acute form of VVC include imbalances in reproductive hormones caused by pregnancy, the use of oral contraceptives, and hormone replacement therapy, as well as the use of large spectrum antibiotics, diabetes, immunosuppression therapy, and stress.8,17 Some data suggest that the local immune response provides important protection during vaginitis.27,31 The vaginal epithelium plays an essential function as an active barrier that protects the female reproductive tract against the pathogenicity of different microorganisms. The expression of immunity receptors in those cells provides sensitivity to different pathogens, and their ability to produce some inflammation mediators leads to the course of local inflammation.4,27
Despite the advances in antifungal therapy, there are many problems related to the available drugs. Some antifungals commonly used to treat VVC, such as azoles, are potentially hepatotoxic. Those drugs have been reported to produce reproductive and developmental toxicity in both humans and laboratory animals.18,23,42 Furthermore, isolates of Candida are reported to be slightly susceptible to one or more azoles.3,40,44 The search for effective compounds with a lower toxicity index leads to investigate natural products as promising candidates in antifungal therapy. The disturbing reality of the progressive emergence of less susceptible strains to antimicrobials also drives this search. A variety of chemical compounds with antimicrobial properties are present in plants, and scientific researches to determine whether those compounds have antibiotic properties against resistant pathogens are necessary.2,20,47
Species of Curcuma are widely found in Asia, and their roots are used in food and traditional medicine.34 Their therapeutic properties are linked to curcuminoids, polyphenolic compounds, being curcumin (1,7-bis[4-hydroxy-3-metoxyphenyl]-1,6-heptadiene-3,5-dione) the most abundant.21 Curcumin is well-known to have therapeutic potential due to its antioxidant, anticarcinogenic, antimutagenic, and antimicrobial activities, among others.48 Some reports have shown that curcumin has antifungal potential against different human pathogens.28,30 Regarding its activity against Candida species, curcumin was shown to be more efficient than fluconazole in the inhibition of oral epithelia fungal adhesion.26 In another study37 it was reported that curcumin acts as an antifungal agent via the generation of oxidative stress, and inhibits hyphae development by targeting TUP1. In addition, the mechanisms of action of curcumin on Candida that have already been described include the inhibition of hyphae development through the action on the TUP1 gene,42 the reduction of ergosterol biosynthesis, the functional modulation of fungal PM-ATPase and the inhibition of fungal exoenzymes SAP (aspartate proteases).24,25 Besides the antifungal effectiveness, there is evidence that curcumin acts as an anti-inflammatory agent by inhibiting cyclooxygenases and lipoxygenases, and also regulates the production of pro-inflammatory cytokines.9,22
Since the pharmacological potential of curcumin is notable, and there is not a robust evidence on the anti-Candidal effect of curcumin in vivo, we investigated the antifungal potential of curcumin and its mechanism of action in vitro and in the therapeutic treatment of VVC in an immunosuppressed rat model.
Materials and methodsCandida strains, animals and drugThree strains belonged to the American Type Culture Collection (C. albicans ATCC 10231, Candida glabrata ATCC 2001, Candida krusei ATCC 34135), and 5 clinical isolates of Candida dubliniensis were obtained from vaginal fluid specimens; the identification was carried out by conventional methods, including growth on chromogenic medium (CHROMagar Paris, France) according to the manufacturer's instructions. The in vivo assay was performed using female Wistar rats (Rattus norvegicus), which were obtained from the Central Animal Facility of Federal University of São João Del-Rei (UFSJ), Campus Dom Bosco. Curcumin (from Curcuma longa, ≥65% of purity), the tested compound in this study, was obtained commercially from Sigma–Aldrich®, Brazil.
Growth conditions and inoculaAll Candida strains were grown on Sabouraud dextrose agar (SDA) (Acumedia®, Brazil) for 48h at 35–37°C. Each inoculum of Candida used in the experiments was standardized to 1×106CFU/ml by adjusting the optical density to 0.08–0.1 at 530nm (Biotek®, Brazil).2C. albicans ATCC 10231 was used for the in vivo infection.
In vitro susceptibility testThe minimum inhibitory concentration (MIC) was determined by the microdilution method according to documents M38-A2 and M27-A3 of the Clinical and Laboratory Standards Institute,10 with minor modifications.1,29,41 The initial concentration of curcumin was prepared at 2000μg/ml (ethanol was used as solvent) and 0.02ml of this solution was added to a 96-well microtitre plate containing Sabouraud dextrose broth (SDB) (Acumedia®, Brazil). The initial test concentration was serially two-fold diluted. Each well was inoculated with 0.1ml of a suspension containing 2.5×103CFU/ml. The antifungals ketoconazole (Sigma–Aldrich®, Brazil) and nystatin (Sigma–Aldrich®, Brazil) were included as positive controls and ethanol (solvent) was included as the negative control. The plates were incubated for 48h at 37°C. The MIC of each compound for every isolate was determined following the addition of 0.05ml of 2% triphenyltetrazolium chloride (TTC, Sigma–Aldrich®, Brazil). Yeast growth was displayed by a change to a red color. MIC was defined as the lowest concentration of compound showing no visible fungal growth after the TTC reaction. The minimal fungicidal concentration (MFC), in turn, was determined as the absence of visible colonies in Sabouraud dextrose agar (SDA), as previously described.19 All experiments were performed in triplicate.
Membrane ergosterol as target of antifungal activityThe MIC of curcumin against C. albicans ATCC 10231, C. glabrata ATCC 2001 and Candida krusei ATCC 34135 was determined following a standard reference method as explained above, in the absence and presence of exogenous ergosterol (200μg/ml) to determine whether curcumin binds to the fungal membrane sterol. If the activity of curcumin was caused by binding to ergosterol, the exogenous ergosterol would prevent curcumin binding to the fungal membrane's ergosterol. As a consequence, MIC values were enhanced in the presence of exogenous ergosterol.15 Nystatin at an initial concentration of 1000μg/ml was used as a control drug. Caspofungin (Sigma–Aldrich®, Brazil), an antifungal that interfere with the fungal cell wall, was used at an initial concentration of 1000μg/ml as a negative control. The MIC was determined after 48h of incubation. All experiments were performed in triplicate.
Experimental VVCFemale Wistar rats (body weight 100–150g) were collectively housed in the experimental room of the Laboratory of Pharmacology of UFSJ-Campus Centro Oeste (25°C±1°C, 12-h dark–light cycle). All applicable national and institutional guidelines for the care and use of animals were followed. Animals were maintained in the environmentally controlled room for at least 5 days before the experiments started. The protocol was approved by the Animal Experimentation Ethics Committee of the Universidade Federal de São João Del-Rei (Protocol 029/2013).
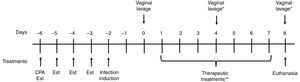
The rat model of vaginal infection was established to obtain a chronic and homogeneous infection.2Fig. 1 presents a schematic representation of the experimental design. Animals were immunosuppressed by the administration of one dose of cyclophosphamide (Sigma®, 50mg/kg b.w.) and estrus was induced by the subcutaneous administration of estradiol cypionate (Pfizer®, Brazil) at a dose of 0.2mg/ml once daily for four days before the infection was induced. Rats were inoculated intravaginally with 0.1ml of C. albicans ATCC 10231 (5×107CFU/ml) using a micropipette with disposable tips. Two days after inoculation (day 0) the vaginal load of C. albicans was evaluated through vaginal lavage with 0.1ml of sterile saline, and determined by the CFU assay on SDA to verify whether the infection had been established. The infection was considered sufficient if a mean count for the vaginal lavage cultures from each rat was at least 103CFU/ml.
Schematic representation of the experimental design. CPA: cyclophosphamide treatment; Est: estradiol cypionate treatment. *The vaginal load of C. albicans was evaluated through vaginal lavage on the fourth and eighth day in order to evaluate the curcumin effectiveness after three and seven days of treatment, respectively **Animals were treated twice per day for seven consecutive days.
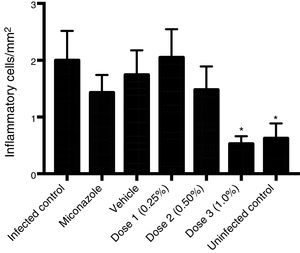
To evaluate the effect of curcumin in VVC, three different concentrations of the compound solution and its controls were administered intravaginally to the infected animals. Wistar females (n=42) were randomized equally into the following seven groups: Group 1: non-infected animals; Group 2: infected animals, not treated; Group 3: infected animals treated with miconazole vaginal cream (0.1ml) (MCZ, 10mg/g, Medley®, Brazil); Group 4: infected animals treated with 0.25% of curcumin solution (0.1ml); Group 5: infected animals treated with 0.5% of curcumin solution (0.1ml); Group 6: infected animals treated with 1% of curcumin solution (0.1ml); and Group 7: infected animals treated with a 20% ethanol solution (vehicle control group) (0.1ml). Treatments were administered to the infected animals twice a day for seven consecutive days. One, four and eight days after the infection was established, the vaginal load of C. albicans was also evaluated to verify the effectiveness of the treatments. On day 8, the animals were euthanized and vaginas were longitudinally removed. All vaginal sections were stained with hematoxylin and eosin (H&E) and observed under light microscopy. From the histological slides stained by H&E, a quantitative analysis of the total inflammatory cells (polymorphonuclear and mononuclear) was performed using a digital image analyzer (Motic Images Plus 2.0 ML); the images were generated by a Zeiss Axiolab microscope connected to a camera (Moticam 580 5.0 MP) interconnected to a computer scanner. The images were obtained from the connective tissue of the vaginal mucosa, where the leucocytes were identified by their characteristic morphology. A total of 10 fields per slide at 400x magnification were analyzed through the Image J Program (NIH, BETHESDA, USA). The results of the quantitative analysis in each group were expressed as the mean±standard deviation of inflammatory cells/mm2.
Statistical analysisTo verify the evolution in the reduction of the vaginal fungal load, analyses comparing the means obtained with curcumin on the third and seventh days with that of the first day after the infection were performed. Data obtained from the groups treated with curcumin were also compared with those from non-infected, infected-not treated groups, and vehicle control group. For the quantitative histological analyses, means between groups were compared. The results were analyzed statistically by the analysis of variance (ANOVA) for completely randomized experiments, with the calculation of the F statistic and the subsequent p values. In the cases in which p value was less than 0.05, treatment means were compared by the Tukey test for multiple comparison analysis between groups, and the minimum significant difference was calculated for α=0.05. GraphPad Prism 7® software was used to perform the statistical analyses.
ResultsAntifungal activity and mechanisms of actionThe MIC value of curcumin against C. glabrata was 125μg/ml, but a concentration of 500μg/ml was required to achieve the same effect for C. krusei. Similar growth inhibition endpoints (1000μg/ml) with C. albicans and the clinical isolates of C. dubliniensis recovered from VVC patients were observed. Table 1 shows the results of MIC and MFC of curcumin with the different Candida species, as well as the MIC values with the antifungal controls (ketoconazole and nystatin).
In vitro antifungal activity of curcumin.
| Candida strains | MIC curcumina | MFC curcumina | MICa ketoconazoleb | MICa nystatinb |
|---|---|---|---|---|
| C. albicans ATCC 10231 | 1000 | >1000 | 62.5 | 3.9 |
| C. glabrata ATCC 2001 | 125 | >1000 | 1.95 | 3.9 |
| C. krusei ATCC 34135 | 500 | >1000 | 0.97 | 7.8 |
| C. dubliniensis clinical isolate 11763 | >1000 | >1000 | 0.97 | ND |
| C. dubliniensis clinical isolate 1083 | >1000 | >1000 | 0.97 | ND |
| C. dubliniensis clinical isolate 1203 | 1000 | >1000 | 0.97 | ND |
| C. dubliniensis clinical isolate 1417 | 1000 | >1000 | 0.97 | ND |
| C. dubliniensis clinical isolate 1252 | 1000 | >1000 | 0.97 | ND |
The results of the action of curcumin on membrane ergosterol indicate that curcumin, as well as nystatin, can bind to C. albicans ergosterol since the presence of exogenous ergosterol increased the MIC values (Table 2), thus suggesting the possible mechanism of action. As expected, caspofungin, the negative control, did not show any MIC increase in the presence of exogenous ergosterol. In this study, the curcumin could not bind to C. krusei and C. glabrata ergosterol since the presence of exogenous ergosterol did not show increased MIC values.
In vitro antifungal activity of curcumin against Candida species with and without exogenous ergosterol.
The immunosuppressed and estrogen-dependent rats were infected with C. albicans, and fungal burden was assessed after 2 days (on day 0). Once the infection was established, we examined the activity of different concentrations of curcumin and MCZ in those animals in which VVC was achieved. On the 3rd day after the beginning of the treatment a new quantification of the fungal burden (through a vaginal lavage) was carried out. In the group of infected and untreated animals and in those treated with solvent ethanol (vehicle control), the fungal burden remained high in five animal out of six during all the study time. In contrast, in the groups treated with different concentrations of curcumin (0.25, 0.5 and 1%) the fungal burden was significantly reduced. On day 7th after the beginning of the treatment, C. albicans was detected in the infected and vehicle control groups at significantly higher levels. The three groups treated with curcumin showed a complete clearance of fungal load on the 7th day after infection (Table 3). The treatment with MCZ exerted the complete clearance of the yeasts on the last day of treatment. Fungal load data are presented in log CFU/ml.
Vaginal fungal burden in all the studied animals according to the different groups.
| Groups | Day 0 | Day 3 | Day 7 | |||
|---|---|---|---|---|---|---|
| Infected animals (%) | LogCFU/ml±SD | Infected animals (%) | LogCFU/ml±SD | Infected animals (%) | LogCFU/ml±SD | |
| Infected control | 6/6 (100) | 3.25±0.20 | 6/6 (100) | 2.04±0.04 | 5/6 (83.3) | 3.00±0.07 |
| Positive control | 6/6 (100) | 4.94±0.06 | 3/6 (50) | 3.89±0.03 | 0/0 (0) | 0.00±0.00* |
| Curcumin 0.25% | 6/6 (100) | 2.46±0.13 | 3/6 (50) | 1.61±0.02* | 0/0 (0) | 0.00±0.00* |
| Curcumin 0.5% | 6/6 (100) | 2.48±0.07 | 0/6 (0) | 0.00±0.00* | 0/0 (0) | 0.00±0.00* |
| Curcumin 1% | 6/6 (100) | 3.53±0.02 | 4/6 (66.6) | 0.49±0.01* | 0/0 (0) | 0.00±0.00* |
| Vehicle | 6/6 (100) | 2.64±0.22 | 6/6 (100) | 2.72±0.72 | 5/6 (83.3) | 3.20±0.27 |
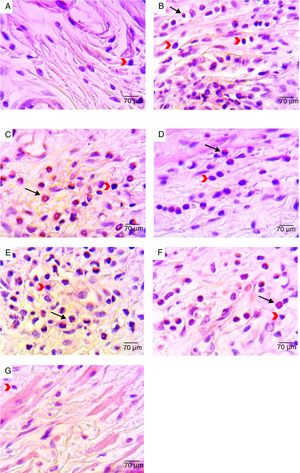
Histological analysis of the vaginal mucosa sections stained with H&E of all the animals was performed by light microscopy; representative images are shown in Fig. 2. We observed histologically normal vaginal mucosa in the uninfected group, while in the infected non-treated group the highest concentration of an inflammatory infiltrate in the lamina propria (below the epithelium) was observed. The histological study of the groups treated with 0.25 and 0.5% curcumin showed inflammatory infiltrate in the vaginal mucosa, with polymorphonuclear infiltration predominantly. The group treated with 1% curcumin showed a reduction in the mucosal inflammatory infiltrate. Regenerative changes associated with the restoration of the vaginal mucosa following MCZ treatment could be seen. Quantitative analysis of sections stained with H&E showed a reduction in the total inflammatory cells (polymorphonuclear and mononuclear) in the group treated with 1% curcumin, with a reduction proportional to the increase in the concentration of curcumin. The group treated with 1% curcumin showed a significant reduction in the number of inflammatory cells when compared to the infected-not treated group (Fig. 3).
Representative photomicrographs of histological analyses of vaginal mucosa sections after different treatments: (A) non-infected animals (negative control) – vaginal mucosa with normal aspect; (B) infected and untreated animals – presence of inflammatory infiltrate in the lamina propria; (C) Infected animals treated with ethanol solution – presence of inflammatory infiltrate in the mucosa with polymorphonuclear infiltration mainly; (D) infected animals treated with MCZ (positive control) – presence of inflammatory process with polymorphonuclear infiltration mainly; (E) Infected animals treated with 0.25% curcumin – inflammatory infiltrate in the vaginal mucosa with polymorphonuclear infiltration mainly; (F) infected animals treated with 0.5% curcumin – inflammatory infiltrate in the vaginal mucosa, with polymorphonuclear infiltration mainly; (G) infected animals treated with 1% curcumin – reduction in mucosal inflammatory infiltrate compared to 0.25% and 0.5% curcumin treatments. The black arrows point to polymorphonuclear leucocytes. The red arrows point to mononuclear leucocytes. H&E stain, 400× magnification.
Curcumin has been evaluated with regard to a variety of biological activities, showing antifungal activity against a number of fungal pathogens, such as Paracoccidioides brasiliensis, Aspergillus, Sporothrix schenckii, as well as different Candida species.24,26,30 Since the current antifungal drugs have limitations, the interest in discovering new and more effective drugs has increased, especially those from natural sources.
The compound exhibited antifungal activity against the three species of Candida included in this study, and fungistatic activity was noted. Curcumin showed activity against C. glabrata at a considerable concentration. An increase in the incidence of invasive candidiasis caused by non-C. albicansCandida species has been observed and it is associated with high mortality and antifungal resistance.40 Among the non-C.albicans Candida species, C. glabrata stands out, having emerged as one of the most important opportunistic pathogens that are particularly capable of infecting a variety of places in the human body; this is the second most frequently isolated species in cases of VVC, preceded only by C. albicans.38,43,46 Several studies have reported the antifungal activity of curcumin. Martins et al.26 showed that curcumin was able to inhibit the growth of several Candida species, and C. albicans was the most susceptible among the species studied. Furthermore, they demonstrated that curcumin dramatically inhibited the adhesion of Candida to epithelial cells. Other studies showed a synergistic effect of curcumin in combination with antifungal agents. Sharma et al. (2010) evaluated the in vitro antifungal effects of curcumin alone as well as in combination with azoles and polyenes against isolates of C. albicans. In wild type C. albicans curcumin showed a synergistic effect in combination with azoles (fluconazole and ketoconazole) and polyenes (amphotericin and nystatin). The MIC80 of curcumin in combination with fluconazole showed a 32-fold reduction, in combination with ketoconazole showed a 64-fold reduction, and a 16-fold reduction was observed when combined with amphotericin B or nystatin. These results demonstrate an important synergetic effect of curcumin in combination with important antifungal agents used in the clinical practice. In a study performed by Neelofar et al.30 curcumin promoted the growth inhibition of C. albicans by using high concentrations of the compound when compared with the effective concentrations of fluconazole. Here, we observed that C. albicans and the clinical strains showed sensitivity to curcumin, with fungistatic activity being observed under the present experimental conditions.
The major sterol component present in the fungal membrane is ergosterol, which plays qualitatively similar properties in fungal membranes as that cholesterol plays in mammalian cell membranes. Ergosterol serves as a bioregulator of membrane fluidity and asymmetry; consequently, the integrity of the cell membrane is dependent on these sterols.5 One of the proposed mechanisms of actions for antifungal agents is binding to membrane ergosterol, which leads to fungal cell disruption and a loss of intracellular content.32 In this work, we observed that the binding to membrane ergosterol might be the mechanism of action of curcumin against fungi. The cell membrane disruption in Candida by means of curcumin has been observed in other studies24; this antifungal activity supports our results. The authors demonstrated that the leakage of potassium ions from the fungal cytosol and a dissipation in the membrane potential was detected by bis-(1,3-dibutyl-barbituric acid) trimethineoxonol [DiBAC4(3)] staining. These authors also investigated an increase in membrane permeability in curcumin-treated C. albicans with an influx of propidium iodide. Fluorescence analysis with 1,6-diphenyl-1,3,5-hexatriene supported the membrane-targeted mechanism of action indicating membrane disruption. Using calcein leakage assays from curcumin-treated large unilamellar vesicles and giant unilamellar vesicles, these authors found that curcumin has a membrane-active mechanism inducing the leakage of intracellular components through the flappy membrane. However, the effect on membrane ergosterol in our study was species-specific; the curcumin was able to bind to ergosterol only in C. albicans ATCC 10231. This fact shows that the binding of curcumin to ergosterol is not a single-action mechanism. The action of curcumin on ergosterol and other fungal cell components should be determined in future studies.
The therapeutic effect of curcumin was examined in an experimental animal model of VVC. It is known that Candida species cause opportunistic infections in hosts with altered physiological or immune responses.46 In general, the development of mucosal infection models requires the use of systemic immunosuppression, which is a valid method for quickly inducing fungal colonization.11 In rodent models, as well as human infection, these are stringently estrogen-dependent.33 In women, VVC is extremely infrequent in the pre-pubertal period, while women become susceptible in the postmenopausal period following hormone replacement therapy. Estrogen exerts a multifunctional permissive role for vaginal candidiasis through a number of both host- and Candida-directed effects.7,39 Since the local immunity is also critical for anti-Candida defense in the vaginal mucosa, estrogen treatment is important because it transforms the epithelium to a pseudo-estrous phase and inhibits local immune defenses, increasing the glycogen content and thereby facilitating mucosal infection.6 Thus, rats must be placed under stable pseudo-estrus conditions to establish experimental vaginal infection; this is usually achieved by estrogen treatment, which is sufficient to allow C. albicans infection to occur. Using the same model, several studies have shown the effectiveness of natural products against C. albicans in the treatment of VVC.2,13,47
As previously reported,2 a C. albicans suspension containing 5×107CFU/ml was sufficient to establish an infection when instilled into the vagina of immunosuppressed-hormone treated rats. Other studies showed that immunosuppressed rats in a prolonged pseudo-estrus state are particularly susceptible to fungal colonization in the vaginal mucosa.2,35 The animals were treated twice a day for seven days and the doses of curcumin were selected on the basis of MIC results and previous in vitro antifungal activity studies using this compound. As shown in Table 3, after the 3rd day, the treatment with curcumin at 0.5% was able to completely clear the fungal burden in all animals, while at 0.25% and 1% the fungal burden was reduced significantly (p<0.05). On the 7th day, the infection was completely cleared in the groups treated with 0.25% and 1% curcumin. On the last day of treatment, the difference was not statistically significant (p>0.05) among the groups treated with curcumin and the one treated with miconazol, but differences were statistically significant when comparing with the infected-not treated and vehicle control groups. The inclusion of control groups was important to prove the test sensitivity and to ensure the curcumin effectiveness, since five animals remained infected in the infected-untreated group and in the vehicle control group during the entire study period.
The histological study of the non-treated group showed signs of tissue reaction related to the infection, since an intense concentration of inflammatory infiltrate was observed. The groups treated with curcumin exhibited a reduction in inflammatory infiltrates, being the degree of reduction proportional to higher concentrations. Some studies have described the anti-inflammatory potential of curcumin and its ability to interact with numerous molecular targets involved in inflammation, including the altered activity of enzymes, such as cyclooxygenases and lipoxygenases, by inhibiting the tumor necrosis factor (TNF) and the pro-inflammatory transcription nuclear factor kappa B (NF-kB).14,16 Debata et al. (2013),12 in a similar experiment with rats, treated the animals intravaginally for three weeks with a cream containing curcumin and then analyzed the results by histological analysis. The authors reported that the vaginal epithelium of the animals did not show any ulceration, areas of necrosis or micro-abscesses, and concluded that the intravaginal application of curcumin in a cream vehicle is feasible and safe.
In conclusion, the in vitro results highlight the potential of curcumin as an effective antifungal against Candida species growth, showing a similar mechanism of action to the commercial antifungal nystatin. Its efficacy in a VVC rat model suggests that curcumin may be an important compound for the treatment of this fungal infection, and that the development of a vaginal cream formulation containing this compound should be considered.
Ethical approvalAll procedures performed in the studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors are grateful to Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) for the financial support which provided fellowships to S.E. Morais and G.F. Figueiredo, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Federal University of São João Del Rei which provided a fellowship to J.T. Andrade.