The filamentous fungus Penicillium roqueforti is a well-known multifunctional cell factory of high added-value biomolecules.
AimsThe objective of this work was to carry out a detailed analysis of the metabolites present in the culture broth of a new marine-derived Penicillium roqueforti strain isolated in the Canary Islands, Spain.
MethodsThe fungal biomass production was carried out in liquid-state fermentation, and after 10–12 days of incubation at 22–25°C, the supernatant mycelia was separated by filtration, and the culture broth (12l) was stored in a refrigerator at 4°C for a subsequent liquid–liquid extraction with dichloromethane (3×), in accordance with the modified Kupchan method. The volatile and semi-volatile organic compounds were separated by chromatography and analyzed using GC–MS and NMR spectroscopy analyses.
ResultsSeveral volatile organic compounds involved in the fatty acid pathway were identified: a terpenoid, a cyclic dipeptide, phthalates, and an alkyl adipate. In addition, three categories of non-volatile compounds (alkanes, fatty acids and 1-alkanols) were identified by spectroscopy. The results show that the fermented broth of this fungal strain has no mycotoxins under the culture conditions applied.
ConclusionsIt is hoped that this chemo-specific information will offer critical input for improving the biotechnological applications of this filamentous fungus.
El hongo filamentoso Penicillium roqueforti es una fábrica celular multifuncional de biomoléculas de alto valor añadido.
ObjetivosEl objetivo de este trabajo fue realizar un estudio minucioso de los metabolitos presentes en el caldo de cultivo de una nueva cepa de Penicillium roqueforti de origen marino aislada en las Islas Canarias, España.
MétodosLa producción de biomasa fúngica se llevó a cabo por fermentación en estado líquido. Tras 10-12 días de incubación a 22-25°C se separó el micelio sobrenadante por filtración y el caldo de cultivo (12 l) se almacenó en un frigorífico a 4°C hasta su posterior extracción líquido-líquido con diclorometano (×3) de acuerdo con el método modificado de Kupchan. Los compuestos orgánicos volátiles y semi-volátiles se caracterizaron por GC-MS y análisis espectroscópico (NMR).
ResultadosSe identificaron varios compuestos orgánicos volátiles implicados en la ruta de los ácidos grasos, un terpeno, un dipéptido cíclico, varios ftalatos y un adipato de alquilo. Además, fueron identificados por espectroscopía tres tipos de compuestos no volátiles: alcanos, ácido grasos y 1-alcanoles. Los resultados mostraron que el caldo fermentado de esta cepa fúngica no presenta micotoxinas en las condiciones de cultivo empleadas.
ConclusionesEs de esperar que esta información quimio-específica aporte datos críticos para el progreso de las aplicaciones biotecnológicas de este hongo filamentoso.
The filamentous fungus Penicillium roqueforti is a hyphomycete used extensively in the dairy industry to add flavor and veining to internally mold-ripened blue cheeses.4 It is a common contaminant mold found in silages, foods and feed.16,42
Although it has been described as a terrestrial fungus, some studies have shown that P. roqueforti strains have high salt tolerance,26,47 with the germination of its spores inhibited only at sodium chloride concentrations of over 100gl−1.18 This extraordinary tolerance to salts, with high osmotic pressures, means that this hyphomycete undergoes adaptations in order to live as a facultative marine fungus.24 Note that average seawater salinity is 35gl−1.
As regards the chemical composition of Penicillium roqueforti, it is worth noting the work on the fatty acid profile of the same together with its lipid metabolism.27,28 Chromatographic studies of the total lipid fraction of P. roqueforti have shown the presence of palmitic, oleic and linoleic acids, sterified in acylglycerides and free fatty acids forms, with the most polar fractions consisting of phospholipids and glycolipids,23 together with free steroids with the skeleton of ergosterol. Further data are provided on the biogenesis of the unsaturated fatty acids depending on the phases of growth.
The applicability of the fungus in food was limited after Wei et al. purified a mycotoxin of the P. roqueforti strain isolated from toxic feed. This substance, elucitaded as PR-toxin, was reported to be lethal for laboratory rats, inhibiting the DNA, RNA and protein biosynthesis.48,49
Moreau et al. also discovered three new metabolites, the Eremofortines A, B and D.35 The chemistry and biological activity of these eremophilanic sesquiterpenes were revised by Moreau,34 and a description was made of the variables that affect the production of the PR-toxin, such as the growth medium factor,10 and/or the degradation of the PR-toxin in P. roqueforti generating the PR amide (Eremofortine E), PR acid, and PR imine.8,9,11
The advances in the description of the secondary metabolism of P. roqueforti noted the configuration of the crystal structure of the enzyme aristolochene synthase,8 the first terpene cyclase enzyme with a fungal origin. Its structure shows that the active centers presumably involved in the cyclization of the farnesyl pyrophosphate leads to the aristolochene, which is the biogenetic precursor of all sesquiterpenes structurally related to the PR-toxin.
Finally, Moreau et al. isolated, from a non-PR-toxin producing strain of P. roqueforti, another mycotoxin described in Botryodiplodia theobromae, the botryodiplodin.36 The authors elucidated its absolute configuration and explained its mutagenic activity.
Traditionally, the taxonomy of the group P. roqueforti was based on its morphological characteristics, on its ability to spread on standard culture media, and on the analysis of its biochemical profiles, establishing the species P. roqueforti var. roqueforti, used in the manufacture of cheese, and P. roqueforti var. carneum.2,13,15,29,40
However, modern molecular genetic tools combined with biochemical profiles have highlighted the need to reclassify the group P. roqueforti, dividing it into three varieties, named as P. roqueforti, P. carneum and P. paneum.5,6,38
The utilization of solid phase micro-extraction techniques, followed by capillary chromatography analysis with a mass detector (GC–MS), has allowed the detection of volatile intermediary sesquiterpenes of the various different metabolic pathways involved in P. roqueforti,12,21 consolidating the hypothesis that, as indicated above, the enzyme aristolochene Synthase (AS) is responsible for cyclizing in P. roqueforti the farnesyl pyrophosphate to aristolochene which, in turn, is considered to be the biogenetic precursor of the PR toxin.
For this reason Demyttenaere et al.,12 Jelen,21 and Calvert et al.7 proposed that together with Germacrene A, Valencene, β-Elemene, β-Gurjunene, α-Chamigrene, α-Panasinsene, β-Patchoulene, α-Selinene, Diepi-α-cedrene, β-Himachalene and β-Bisabolene, the profile of volatile components should be considered to allow the chemical and taxonomic differentiation of the PR toxin producer strains from those that can not biosynthesize PR toxin. The authors emphasized that these chemical markers, detectable by GC–MS, were absent in the non-toxic strains of Penicillium roqueforti.
The identification of the secondary metabolites may be of assistance in the task of taxonomic differentiation although, since the biogenic routes are dependent on the culture conditions, the results should be considered with caution.17,19,31,43,44
This is the reason why Jelen21 studied the influence of the culture conditions (temperature and water content of the broth) and concluded that, although these may influence the amount of sesquiterpenes produced or the quantitative analysis, their profile remains intact as does the qualitative analysis, which is unique and characteristic of the toxic strains.
Recently, new data have been provided from the cultivation of a strain of P. roqueforti derived from the marine environment, with respect to the volatile components involved in the pathway of fatty acids, along with the fragments produced as a result of catabolism, together with the terpenoids and metabolites that pertain to the shikimic acid pathway. Furthermore, some components, such as triolein, ergosterol peroxide, 9(11)-dehydroergosterol peroxide, 4-hydroxybenzaldehyde and D-mannitol, may be characterized by spectroscopy.33 Note that no chemical component related to the mycotoxins from the toxic strains was identified.
When attempting to give a comprehensive study of the metabolites biosynthesized by this fungus, a detailed investigation of the culture broth was carried out, where the volatile and semi-volatile organic compounds were characterized, using GC–MS and NMR spectroscopy analysis. It is to be expected that this chemo-specific information may provide critical input for improving the biotechnological applications of this filamentous fungus.
Material and methodsIsolation and identification of the fungusThe halotolerant fungal strain was obtained from seawater collected at the intertidal zone of “La Laja” beach, Gran Canaria, the Canary Islands, Spain. The isolation process and strain purification were carried out in Petri dishes on a modified KMV solid medium consisting of 1g yeast extract, 1g hydrolyzed gelatin, 1g peptone, 5g glucose and 12g of bacteriological agar in 1 l of filtered seawater (35 g l–1 salinity). The fungus was identified, using morphological criteria as defined by CABI Bioscience, Surrey, UK (see supplemetary material), as P. roqueforti, and a voucher specimen was deposited at the microbiology strain collection of the Chemistry Department of the University of Las Palmas de Gran Canaria for future reference under the accession number PA 002.
Fungal fermentationThe fungal biomass production was carried out in liquid-state fermentation, in static polypropylene boxes (83×46×18cm) previously sterilized with sodium hypochlorite, and steamed for 5min. After the steam had condensed, the water was drained out of the boxes, and the culture broth was introduced with the previously inoculated strain. The liquid medium used for the fungal production was the KMV modified broth made from 1g yeast extract, 1g hydrolyzed gelatin, 1g peptone, and 5g glucose in 1l of filtered seawater (35g l–1 salinity), which was sterilized by autoclaving (20min) and distributed over several boxes (1.2l/unit; See supplementary material). After 10–12 days of incubation at 22–25°C, the supernatant mycelia was separated by filtration, and the culture broth (12 l) was stored in a refrigerator at 4°C for a posterior liquid-liquid extraction with dichloromethane (3×), in accordance with the partition scheme described in supplementary material.
Apparatus and analytical methodsNormal-phase chromatography was carried out on silica gel (Scharlau) with 0.06–0.2mm particle size for the adsorbent and 0.04–0.06mm for the stationary phase. Chromatography was performed either at medium pressure (Büchi Chromatography System) or at low pressure with a Fluid Metering Inc. motor connected in series with an Ace Glass Inc. column. Reverse-phase chromatography was carried out on a LiChroprep RP-18 (40–63μm particle size, Merck) column connected to a low-pressure chromatography system based also on a Fluid Metering Inc. apparatus.
Size-exclusion chromatography was carried out on lipophilic Sephadex® LH-20 (Sigma). The column was eluted, first, with anhydrous methanol (2h) and, then, with a mixture of CH2Cl2/CH3OH (50:50, 2h). The extracts were applied at the top of the column and eluted with CH2Cl2/CH3OH (50:50) at a rate of 1.0mlmin−1.
Normal-phase TLC was performed on silica gel plates (0.25mm diameter, Tracer Analitica) using a combination of n-hexane, ethyl acetate, chloroform and methanol as an eluent, in the proportions detailed for each case. Reverse-phase TLC was carried out on RP-18F254 plates (0.25mm, Merck) with the use of CH3CN/CH3OH/H2O (80:18:2) as a mobile phase. In each and every one of the cases, the spots were revealed by spraying them with oleum (sulphuric acid, 4%+acetic acid, 80%+water, 16%) and heating thereafter at 120°C for 20min.
Normal-phase semi-preparative HPLC was performed using an Alltech Econosphere silica column (10μm particle size, 250 x 4.6mm, 100Å pore size) and reverse-phase semi-preparative HPLC on a Waters ODS column (10μm particle size, 250×4.6mm, 100Å pore size). Both of these processes were carried out with a semi-preparative HPLC apparatus coupled up to Spectra-physics P100 isocratic pump and used in line with a Hewlett Packard 1050 UV–vis variable wavelength detector, working at room temperature (26°C).
Analytical chromatography was performed using a Shimadzu HPLC system with an LC-9A pump connected in line with an UV SPD-6AV detector (254nm). The condition used for the normal-phase column was a combination of n-hexane and ethyl acetate as an eluent, and in the case of the size-exclusion chromatography column (Shodex OH Pak SB 806 HQ), a mixture of water and 0.05% of sodium azide was used as an eluent. An eluent flow rate of 1.0mlmin−1 was used in all the analyses.
1H, 13C, and 2D NMR experiments recorded at 250 or 300MHz on AC or AMX Bruker apparatus, respectively. A Varian UNITY INOVA 400MHz NMR spectrometer was used for high resolution analysis. Tetramethylsilane was used as an internal standard for 1H and deuterated chloroform (δ 77.00) or deuterated methanol (δ 49.00) for the calibration of the 13-carbon NMR spectra.
Electrospray ionization mass spectrometry was performed either at low or at high resolution with a common electron impact mass spectrometer (IE) or by fast atom bombardment (FAB). Positive mode was carried out on a FAB-MS at 70eV with a FISONS VG Micromass Autospec apparatus with NBA (3-nitrobencylic alcohol) as the matrix.
Gas chromatography–mass spectrometry (GC–MS) analysis was carried out on a chromatograph model Varian CP3800 with an ion-trap mass spectrometer, model Saturn 2000, under the following conditions: CP-Sil 8 low bleed/MS capillary column. The injector temperature was kept isothermal at 270°C; initial split conditions on; 0.01min off and 5min on with a split ratio 1:50; the oven was set at 50°C for 5min, and then ramped at 15°Cmin−1 to 250°C and held for 10min (total run time of 28.33min for each sample); flux of 1ml min−1; mass detector in the EI mode (the m/z range was 20–400).
Compounds lacking reference standard were quantified using the response factor for alkanes (Dr. Ehrenstorfer GmbH Alkanes-Mix 10), fatty acid methyl esters (SupelcoTM 37 Component FAME Mix), 1-alkenes (Fluka Chemika) and 1-alkanols (Fluka Chemika). Rest of the compounds were assigned by structural analogy to the above. Thus, the hexadecanoic acid octadecyl ester (9) was assigned to the factor obtained experimentally for the hexadecanoic acid methyl ester (764.117×10−12mg/Kcounts). The same factor was assigned to the cycle-ProLeu dipeptide (14) and to the 10-undecenoic acid methyl ester due to the resemblance in molecular size and number of double bonds.
Results and discussionChemical analysisThe fermented broth of P. roqueforti was fractioned using CH2Cl2 (3×) to give 175mg of a light brown residue, which was subjected to the partitioning scheme described in the supplementary material. All the fractions were screened carefully, both the volatile and non-volatile compounds, and the following substances were identified and quantified.
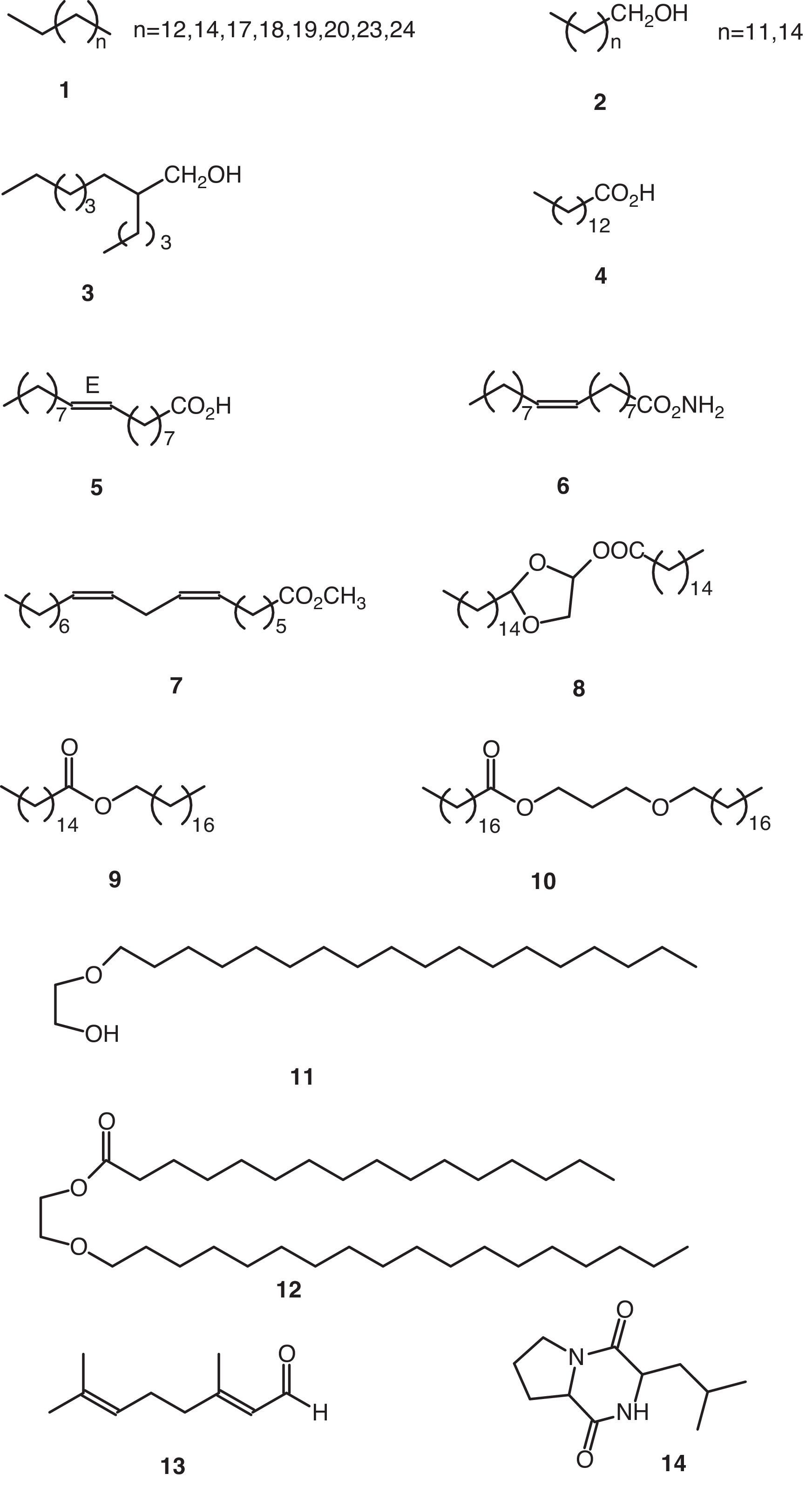
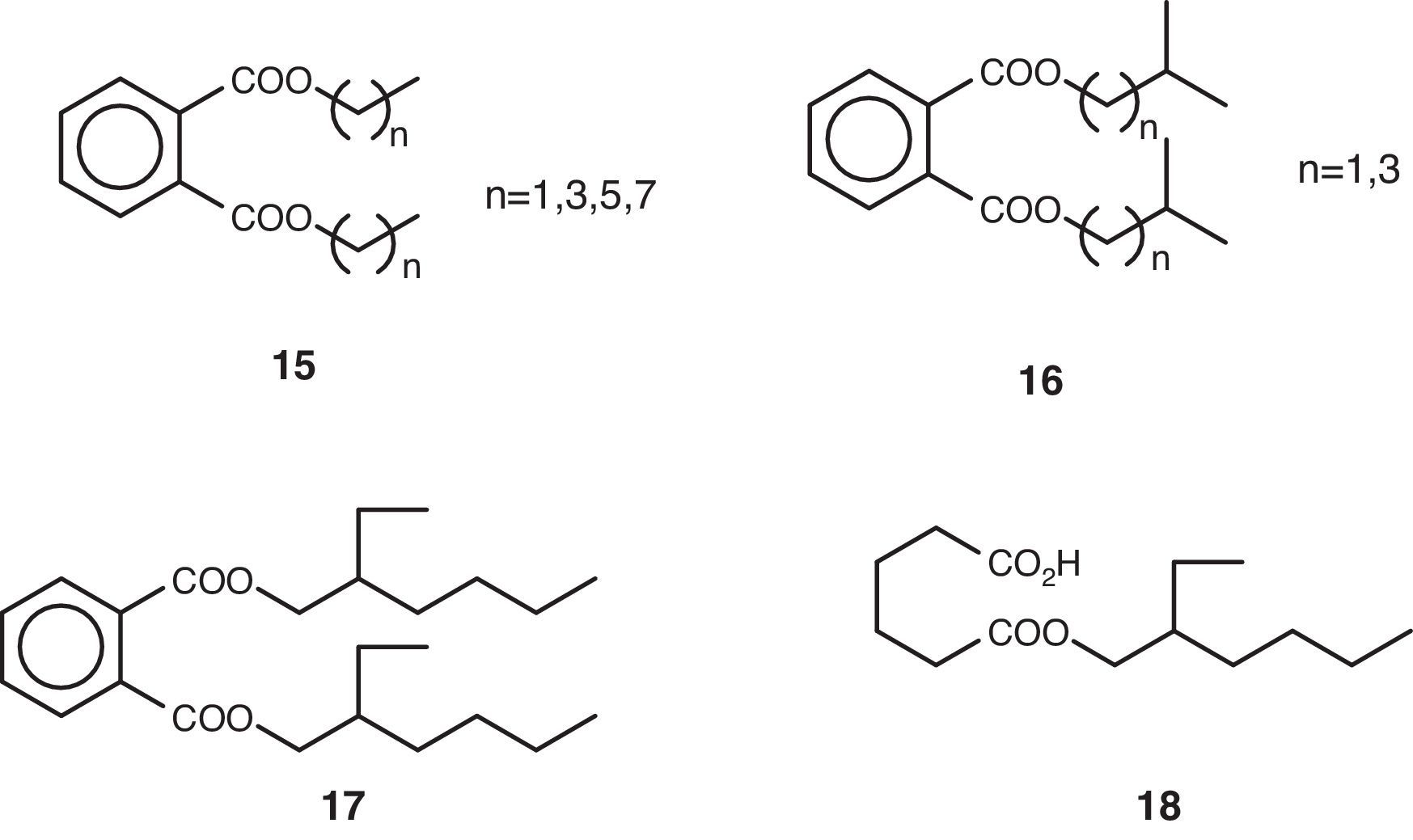
Volatile compoundsThe volatile organic compounds were characterized by GC–MS (Table 1). These substances were grouped together on account of their structural criteria (Figs. 1 and 2) with the following compounds identified and quantified: n-alkanes (1), 1-alkanols (2), one 2-alkyl-1-alkanol (3), saturated (4) and unsaturated (5) free fatty acids, one fatty acid amide (6), one poly-unsaturated fatty acid methyl ester (7), one monoglyceride (8), wax-acid esters and fatty alcohols (9, 10 y 12), one free fatty alcohol (11), one straight-chain monoterpene (13), one cyclic dipeptide (14), alkyl phthlates (15–17), and one alkyl adipate (18).
GC–MS analysis of the volatile organic compounds identified/quantified in the fermented broth of Penicillium roqueforti.
| N° | Rt (min) (mean±SD) | Compound (structurea); concentration (mg/l)×103 |
|---|---|---|
| 1 | 12.166±0.012 | Hexadecanoic acid (2-pentadecyl-1,3-dioxolan-4-yl)-methyl ester (8); 0.73 |
| 2 | 12.179±n.d. | 2,6-Octadienal, 3,7-dimethyl-, (E)-(13); 6.66 |
| 3 | 12.524±0.007 | 2-Butyl-1-octanol (3); 0.21 |
| 4 | 13.991±0.001 | Pentadecane (1, n=12); 3.50 |
| 5 | 14.771±0.005 | Tetradecanoic acid (4); 0.52 |
| 6 | 14.857±0.010 | 1-Tridecanol (2, n=11); 0.19 |
| 7 | 15.063±0.001 | Diethyl phthalate (15, n=1); 0.69 |
| 8 | 15.642±0.000 | Heptadecane (1, n=14); 2.50 |
| 9 | 16.338±0.010 | 1-Hexadecanol (2, n=14); 0.25 |
| 10 | 17.085±0.005 | Bis(2-methylpropyl) phthalate (16, n=1); 1.39 |
| 11 | 17.393±n.d. | Eicosane (1, n=17); 5.25 |
| 12 | 17.560±n.d. | Cyclo(Pro-Leu) (14); 17.58 |
| 13 | 17.653±0.009 | Dibutyl phthalate (15, n=3); 543.29 |
| 14 | 17.948±n.d. | Stearic acid, 3-(octadecyloxy)-propyl ester (10); 0.20 |
| 15 | 18.327±n.d. | Heneicosane (1, n=18); 11.75 |
| 16 | 18.360±0.008 | 9-Octadecenamide (6); 5.58 |
| 17 | 18.378±n.d. | 7,10-Octadecadienoic acid, methyl ester (7); 14.83 |
| 18 | 18.646±n.d. | Hexadecanoic acid, octadecyl ester (9); 20.92 |
| 19 | 18.978±n.d. | Docosane (1, n=19); 11.83 |
| 20 | 19.048±0.058 | Hexadecanoic acid, 2-(octadecyloxi)-ethyl ester (12); 9.92 |
| 21 | 19.748±0.005 | Tricosane (1, n=20); 20.83 |
| 22 | 19.810±0.004 | 9-Octadecenoic acid, (E)-(5); 6.47 |
| 23 | 20.407±n.d. | Bis(4-methylpentyl)-phthalate (16, n=3); 80.67 |
| 24 | 20.714±n.d. | 2-Ethylhexyl adipate (18); 11.92 |
| 25 | 21.805±n.d. | 2-(Octadecyloxi)-ethanol (11); 46.25 |
| 26 | 22.648±0.003 | Bis(2-ethylhexyl)-phthalate (17); 17.83 |
| 27 | 22.746±0.013 | Dihexyl phthalate (15, n=5); 243.25 |
| 28 | 23.242±n.d. | Hexacosane (1, n=23); 147.17 |
| 29 | 25.070±n.d. | Heptacosane (1, n=24); 49.00 |
| 30 | 26.390±0.023 | Dioctyl phthalate (15, n=7); 195.75 |
Rt=retention time. n.d.=no date.
The non-volatile material obtained from the culture broth was fractioned using a normal-phase chromatography column (silica gel), size-exclusion chromatography (LH-20 lipophilic) and normal-phase semi-preparative HPLC. The fractions obtained were analyzed by spectroscopy, finding that they consisted primarily of high molecular weight alkanes (1), free fatty acids, 1-alkanols (2) and dinonyl phthalate (15, n=8). These fractions showed the following spectroscopic data: the alkanes {1H-NMR (Cl3CD) δ 0.90 (3H, t, J=7Hz), 1.28 (24H, m); MS 366.42 (9%)}; the fatty acids {1H-NMR (Cl3CD) δ 0.90 (3H, t, J=7Hz), 1.28 (37H, m), 1.65 (2H, m), 2.36 (2H, t, J=7Hz); MS 340.34 (9%)}; the 1-alkanols {1H-NMR (Cl3CD) δ 0.88 (3H, t, J=7Hz), 1.28 (27H, m), 1.54 (2H, m), 3.64 (2H, t, J=7Hz); MS 224.25 (2.5%)}; and dinonyl phthalate {1H-NMR (Cl3CD) δ 0.89 (6H, t, J=7Hz), 1.34 (24H, m), 1.74 (4H, m), 4.32 (4H, t, J=7Hz), 7.64 (4H, m); MS 418.31 (1.5%)}. Note that some of these compounds described in the previous section have also been described as volatile components.
Absence of toxinsAttempts to find metabolites involved in the biogenetic PR-toxin route of the culture broth used were, therefore, unsuccessful, as they did not detect any of the volatile chemical tracers proposed by Demyttenaere et al.12, Jelen21 or Calvert et al.7 Consistent with this observation, no single non-volatile organic compound was found that could bind biogenetically with the PR-toxin, or with botryodiplodin.
Presence of a cyclic dipeptideHowever, it should be noted here that there was identification of cyclo-ProLeu {pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-isobutyl} (14). This substance, which had been previously isolated from both peptone25 and various fungal broths,46 belongs structurally to the family of the cyclic dipeptides that exhibit a broad spectrum of biological activity. Thus, the same cyclo-ProLeu (14) has been proposed as a bitter-tasting food additive with antifungal activity,46 the cyclo-ProTyr and the cyclo-ProPhe, initially described as phyto-toxins of the fungus Alternaria alternata,46 have been marketed as bio-rational pesticides due to their high stability and low toxicity for mammals, meaning that the herbicide Scythe® and others have been patented for their anti-inflammatory and anti-allergic properties.3,45
Hydrocarbons and free fatty acids in culture brothSome marine organisms use simple hydrocarbons or fatty acids, which are practically insoluble in seawater, as chemical signals.39 In some cases, these act by way of sex pheromones32 and, in others, through allelopathic factors.22 It is no surprise, then, that n-alkanes (1) and free fatty acids (4, 5) were identified in the culture broth.
Phthalates and alkyl adipatesThe bis-(2-ethylhexyl) phthalate (17) has been proposed previously in the literature as an authentic metabolite in the fungus Penicillium olsonii1 and even given the consideration of an agent with anti-amyloidogenic and neuro-protective activity.
In fact, dibutyl phthalate (15, n=3) and the bis(2-ethylhexyl) phthalate (17) have been described as being cathepsin B inhibitors.20 Cathepsin B is a protease that plays an important role in natural defences against Alzheimer's disease.37 In addition, this substance, which is known under its international status of DEHP {di(2-ethylhexyl) phthalate} or as DOP (dioctyl phthalate), is widely used in plasticides such as PMMA {poly(methyl methacrylate) and PVC {poly(vinyl chloride)}. The 2-ethylhexyl adipate (18), on the other hand, is a DEHA derived product {di(2-ethylhexyl) adipate}.
These substances migrate from plastics and enter into biological fluids where they are bio-accumulated over time, a phenomenon which is known to cause health problems in human beings who have been exposed to the said plastics.14 That is why the identified substances 15–18 in the culture broth of P. roqueforti raise questions with respect to their origin, because the mass cultivation was carried out in plastic containers (see experimental).
MacKenzie et al.,30 working with extracts obtained from the mycelium of the marine fungus Monodictys pelagica, are categorical in stating that these phthalates are actually artifacts, and are by no means metabolites of fungal origin as it had been previously stated in the literature. Whereupon, the biotechnological novelty is that, possibly, this fungus plays a part in the degradation of the polyvinyl acetate found in the culture boxes, which constitutes a phenomenon to be observed in other hyphomycetes, such as Aspergillus parasiticus, Fusarium subglutinans and Penicillium funiculosum.41
ConclusionsA total of 30 volatile organic compounds were identified and quantified in the marine-derived P. roqueforti culture broth. These were n-alkanes, 1-alkanols, one 2-alkyl-1-alkanol, saturated and unsaturated free fatty acids, one fatty acid amide, one poly-unsaturated fatty acid methyl ester, one monoglyceride, wax-acid esters and fatty alcohols, one free fatty alcohol, one straight-chain monoterpene, one cyclic dipeptide, alkyl phthlates, and one alkyl adipate. Additionally, four categories of non-volatile components were also identified by spectroscopy: alkanes, fatty acids, 1-alkanols and dinonyl phthalate.
The results have shown that the fermented broth of this fungal strain has no toxins, which means that this strain of P. roqueforti has potential applications in nutraceuticals and in the production of bio-molecules.
Conflict of interestThe authors declare no conflict of interest.
The authors thank the CAPES agency (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil) for the PhD fellowship and the European Commission for a Marie Curie Training Site Fellowship, both granted to R.M. Grateful acknowledgment is made of the financial support given to the project SI-697 (ULPAPD-08/01-5) by the Canary government (Agencia Canaria de Investigación, Innovación y Sociedad de la Información, ACIISI), and the ICIC (Instituto Canario de Investigación del Cáncer). We also express our thanks to Margaret J.H. Robertson for language correction.