An allogeneic hematopoietic cell transplantation (allo-HCT) patient presented with chronic pulmonary aspergillosis associated to pulmonary graft versus host disease (GVHD) and was treated for a long time with several antifungal agents that were administered as prophylaxis, combination therapies, and maintenance treatment. The patient suffered from a breakthrough invasive pulmonary aspergillosis due to Aspergillus fumigatus after long-term antifungal therapy.
Material and methodsSeveral isolates were analyzed. First isolates were susceptible in vitro to all azole agents. However, after prolonged treatment with itraconazole and voriconazole a multiple azole resistant A. fumigatus isolate was cultured from bronchoalveolar lavage (BAL) when the patient was suffering from an invasive infection, and cavitary lesions were observed.
ResultsAnalysis of the resistant mechanisms operating in the last strain led us to report the first isolation in Spain of an azole resistant A. fumigatus strain harboring the L98H mutation in combination with the tandem repeat (TR) alteration in CYP51A gene (TR-L98H). Long-term azole therapy may increase the risk of resistance selecting strains exhibiting reduced susceptibility to these compounds. However, since the isolates were genetically different the suggestion that could be made is that the resistance was not induced during the prolonged azole therapy but the patient might simply have acquired this resistant isolate from the environment, selected by the therapy.
ConclusionsThese findings suggest that in all long-term treatments with antifungal agents, especially with azoles, repeated sampling and regular susceptibility testing of strains isolated is necessary as resistant isolates could be selected.
Un paciente sometido a un trasplante alogénico de progenitores hematopoyéticos se presentó con aspergilosis pulmonar crónica, asociada a enfermedad pulmonar injerto contra huésped, y fue tratado durante un período prolongado con diversos antimicóticos administrados como profilaxis, tratamiento de combinación y tratamiento de mantenimiento. El paciente experimentó una aspergilosis pulmonar invasiva recurrente debida a Aspergillus fumigatus tras el tratamiento antimicótico prolongado.
Material y métodosSe analizarán diversos aislamientos. Los primeros aislamientos eran sensibles in vitro a todos los azoles. No obstante, tras tratamiento prolongado con itraconazol y voriconazol, a partir de líquido de lavado broncopulmonar (LBA) cuando el paciente experimentó una infección invasiva, se cultivó un aislamiento de A. fumigatus resistente a múltiples azólicos y se observaron lesiones cavitarias.
ResultadosEl análisis de los mecanismos de resistencia que actuaron en la última cepa nos condujo a publicar un artículo sobre el primer aislamiento en España de una cepa de A. fumigatus resistente a un azol que albergaba la mutación L98H en combinación con una alteración de repeticiones en tándem (RT) en el gen CYP51A (TR-L98H). El tratamiento prolongado con un azol puede aumentar la selección de cepas con una disminución de la sensibilidad a estos fármacos. Sin embargo, en este caso, puesto que los aislamientos eran genéticamente diferentes, se sugirió que la resistencia no estuvo inducida durante el tratamiento prolongado con azólicos sino que el paciente adquirió este aislamiento resistente del entorno y fue seleccionado por el tratamiento.
ConclusionesLos hallazgos del presente estudio sugieren que en todos los tratamientos crónicos con antimicóticos, en particular con azólicos, puede ser necesaria la obtención de muestras repetidas, al igual que la realización de exámenes a intervalos regulares de la sensibilidad de las cepas aisladas, ya que pueden seleccionarse aislamientos resistentes.
A 36-year-old woman presented pulmonary aspergillosis due to multiple azole resistant Aspergillus fumigatus. The patient suffered from chronic myeloid leukemia (CML) Ph+, diagnosed in September 2001, and was initially treated with hydroxiurea and interpheron alpha 2b. On August 9th 2002, in the first chronic phase of CML, she underwent allogeneic hematopoietic cell transplantation (allo-HCT) from her HLA identical sister, with oral busulfan plus cyclophosphamide (BUCY) as conditioning regimen, and cyclosporine plus methotrexate as prophylaxis for graft versus host disease (GVHD). Antifungal prophylaxis was not prescribed at that time. The patient was discharged on August 30th 2002.
In September 2002, she was readmitted due to acute cutaneous GVHD which was treated with systemic corticotherapy. Cytomegalovirus (CMV) antigenemia was positive and ganciclovir was prescribed. In addition, fluconazole (100mg/d) was administered for 30 days after diagnosis of oral candidiasis. From October 2002 to March 2003, the patient was treated with antibacterial agents due to several episodes of respiratory infections. In January 2001 a diagnosis of pulmonary GVHD was made. On March 6th 2003, oral itraconazole (100mg/d) was added to treat a relapse of oral candidiasis. Later, on March 17th, she was treated for respiratory infection with dyspnea. A chest computed tomography showed bilateral pulmonary nodules and a cavitary lesion with halo-sign (3–4cm) in right upper lobe. The galactomannan (GM) antigen quantification was positive (index>0.7) in four determinations done on March 28th, April 1st, 4th and 8th of 2003. Pseudomonas aeruginosa, Enterobacter cloacae and A. fumigatus (1st fungal isolate, identified as CNM-CM-2495, Filamentous Fungi Collection of Spanish Center for Microbiology) were isolated from sputum specimens. She was classified as having probable aspergillosis1 and treated for ten days with liposomal amphotericin B (5mg/kg/d), substituted later for caspofungin (70mg/d as loading dose and then 50mg/d) plus intravenous voriconazole (6mg/kg q12h for the first 24h and then 4mg/kg q12h) due to intolerance to the polyene. After 35 days of antifungal combined therapy and upon clinical improvement (two GM negative determinations), the patient was discharged with oral voriconazole (200mg q12h) as maintenance therapy.
From May to October 2003, she was readmitted three times because of impaired respiratory function due to pulmonary GVHD. P. aeruginosa and E. cloacae were isolated again from several sputa. Treatment with corticoids and antibacterial agents was prescribed. Oral voriconazole was maintained at a dosage of 200mg/12h and fungi were not isolated. Tomography showed persistence of lung cavitary nodules.
In October 2003 she was treated for pneumonia and P. aeruginosa and A. fumigatus were isolated again, this time from bronchoalveolar lavage (BAL) (2nd fungal isolate, CNM-CM-2627). She continued with voriconazole (200mg/12h) and GM quantification was 0.7. From October 2003 to February 2004, the patient remained in serious condition with respiratory insufficiency, cavitary lesions in right lung and GM positive. On February 5th 2004, Aspergillus nidulans (3rd fungal isolate, CNM-CM-2797) was isolated from sputum. Voriconazole treatment was maintained.
In March 2004, given the persistency of respiratory infection symptoms, voriconazole was discontinued and nebulized liposomal amphotericin B was prescribed (25mg three times a week for the two first weeks, then 25mg once a week). In May 2004, the respiratory symptoms worsened and the polyene was changed to a combination of caspofungin and voriconazole at standard doses for 25 days. GM was positive again with indexes at around 1.5. The patient was discharged on May 27th with nebulized liposomal amphotericin B as maintenance therapy, changed to oral voriconazole (200mg/q12) in September 2004. The patient was evaluated for a lung transplant, but her respiratory function deteriorated and she died in March 2005 before undergoing transplant.
In summary, an allo-HCT patient presented a chronic pulmonary aspergillosis associated to a pulmonary GVHD. She was extensively treated with antifungal agents including fluconazole (30 days), itraconazole (25 days), voriconazole (16 months), amphotericin B (9 months) and caspofungin (60 days). Antifungal compounds were administered as prophylaxis, combination therapies, and maintenance treatment.
Microbiological and molecular proceduresThe clinical isolates described in the case report were recovered from a patient with chronic pulmonary aspergillosis associated to chronic pulmonary GVHD. The first isolate (CNM-CM2495) was susceptible in vitro to azole agents. However, after prolonged treatment with itraconazole (one month) and voriconazole (8 months), a second isolate resistant to azole agents was cultured from BAL when the patient was suffering from a new episode of respiratory infection, and cavitary lesions were observed in right lung. Four months later, and after twelve months of voriconazole therapy, A. nidulans with azole cross-resistance was isolated from sputum when the patient showed renewed signs of respiratory infection.
The three fungal isolates were submitted to the Spanish National Center for Microbiology for species identification and susceptibility testing. There, strains were labeled as CNM-CM-2495, CNM-CM-2627, and CNM-CM2797 and subcultured at 24°C and at 40°C on malt extract agar (MEA 2%, Oxoid Unipath, Madrid, Spain) and at 37°C on brain–heart infusion (BHI, Oxoid). After 7 days, macroscopic and microscopic examinations were done.2 Isolates CNM-CM-2495 and CNM-CM-2627 were identified as A. fumigatus and CNM-CM2797 as A. nidulans. The isolates were analyzed genetically to confirm identification. They were cultured on YEPD medium (0.3% yeast extract, 1% peptone, 2% dextrose) and grown overnight at 24°C. Mycelial mats were recovered and subject to a DNA extraction protocol. DNA segments comprising the region ITS1 and ITS2, and part of the beta-tubulin gene were amplified and sequenced.3,4 The sequence analysis was performed by comparing with the nucleotide sequences. For these analyses we used the sequence database at the Spanish Mycology Reference Laboratory, which holds 5000 strains belonging to 270 different fungal species. This database was designed by the Spanish National Center for Microbiology and has restricted access. The clinical strains were confirmed as A. fumigatus and A. nidulans.
Susceptibility testing was performed by microbroth dilution following the AFST-EUCAST reference method.5 Amphotericin B (ranged 16.0–0.03μg/mL, Sigma Aldrich Quimica S.A., Madrid, Spain), itraconazole (8.0–0.015μg/mL, Janssen S.A., Madrid, Spain), voriconazole (8.0–0.015μg/mL, Pfizer S.A., Madrid, Spain), posaconazole (8.0–0.015μg/mL, Schering-Plough, Kenilworth, NJ, USA), caspofungin (16.0–0.03μg/mL, Merck & Co., Inc., Rahway NJ, USA), micafungin (16.0–0.03μg/mL, Astellas Pharma Inc., Tokyo, Japan), and anidulafungin (16.0–0.03μg/mL, Pfizer S.A.) were tested. For testing echinocandins against molds, the MIC value was defined as the lowest drug concentration resulting in aberrant hyphal growth by examination with an inverted microscope, that is, the minimum effective concentration (MEC). Susceptibility tests were performed three times with each strain on different days. Aspergillus flavus ATCC 204304 and A. fumigatus ATCC 204305 were used as quality control strains for susceptibility testing.2,6,7 To define susceptibility and resistance in vitro, the criteria used were according to the epidemiological cut-off values (ECVs), recently published for A. fumigatus. For itraconazole, and voriconazole, the wild-type populations were defined as isolates with MIC values ≤1μg/mL and for posaconazole ≤0.25μg/mL.8,9
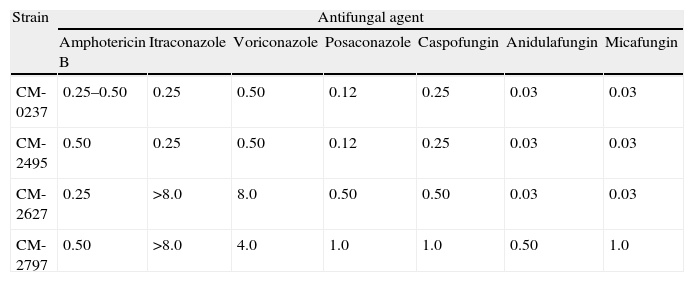
Results and discussionTable 1 shows the MIC values for the three isolates analyzed. Amphotericin B and echinocandins exhibited a good activity against all of them. However, azole agents had different activity in vitro. The first isolate (CNM-CM-2495 dated in March 2003) was susceptible in vitro to itraconazole, voriconazole (MIC≤1mg/L), and posaconazole (MIV value≤0.25mg/L). The second isolate (CNM-CM-2627), obtained in October 2003 after 8 months of voriconazole therapy, was resistant in vitro to itraconazole, voriconazole and posaconazole, and the third (CNM-CM-2797), cultured after 12 months of voriconazole therapy, exhibited MIC values over the ECVs set to interpret AST results.8
Susceptibility testing results of the Aspergillus spp. clinical strains and the A. fumigatus (CM-0237) wild type reference strain.
| Strain | Antifungal agent | ||||||
| Amphotericin B | Itraconazole | Voriconazole | Posaconazole | Caspofungin | Anidulafungin | Micafungin | |
| CM-0237 | 0.25–0.50 | 0.25 | 0.50 | 0.12 | 0.25 | 0.03 | 0.03 |
| CM-2495 | 0.50 | 0.25 | 0.50 | 0.12 | 0.25 | 0.03 | 0.03 |
| CM-2627 | 0.25 | >8.0 | 8.0 | 0.50 | 0.50 | 0.03 | 0.03 |
| CM-2797 | 0.50 | >8.0 | 4.0 | 1.0 | 1.0 | 0.50 | 1.0 |
Data are MIC values in mg/L.
The analysis of this case study was completed retrospectively assessing the mechanism of resistance at a molecular level based on previous reports.10–14 The typing the two A. fumigatus clinical isolates was achieved using a reliable procedure.15–17
The analysis of resistance at a molecular level was done by DNA isolation and cyp51A and cyp51B genes PCR amplification. Conidia from each strain were inoculated into 3mL of GYEP broth (2% glucose, 0.3% yeast extract, 1% peptone) and grown overnight at 37°C. Mycelia mats were recovered and subject to a DNA extraction protocol.12 The full coding sequences of both cyp51 genes and the cyp51A promoter were PCR amplified and sequenced as previously described.11,13,14 To rule out the possibility that any sequence change identified was due to PCR-induced errors, each strain was independently analyzed twice. The AF293 isolate, a strain susceptible to antifungal compounds and used for the sequencing of A. fumigatus genome, and the CNM-CM-237 isolate, a clinical strain fully susceptible to antifungal agents, were used as control strains.
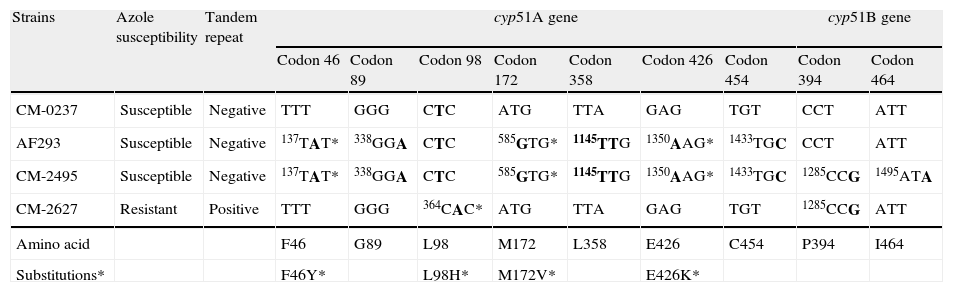
Sequence analysis of cyp51s revealed a number of point mutations shown in Table 2. The first clinical isolate (CNM-CM-2495) had six extra base changes in cyp51A (t137a, g338a, a585g, a1145g, g1350a, t1433c), some of which were responsible for amino acid changes compared to the sequence of control strain CNM-CM-237. However, all of these mutations were present in the azole susceptible control strain AF293, and consequently these changes could be interpreted as genetic polymorphisms without significance for the susceptibility profile of the isolate.18 The second strain, CNM-CM-2627, was azole resistant and a t364a point mutation was detected. It resulted in an amino acid substitution of leucine for histidine (L98H) at codon 98 accompanied by a 34-bp (5′-GAATCACGCGGTCCGGATGTGTGCTGAGCCGAAT-3′) duplication in tandem, located in the cyp51A promoter at positions 288 and 322 from the cyp51A start codon. This change has been described and classed as one of the most common mechanisms of resistance to azoles in A. fumigatus.19–22 In addition, the two clinical strains also contained three different point mutations in the cyp51B gene that were not conserved in the two isolates. These changes were considered silent according to previous studies reported.11–14
Nucleotide and amino in cyp51A and cyp51B genes from A. fumigatus clinical isolates and control strains.
| Strains | Azole susceptibility | Tandem repeat | cyp51A gene | cyp51B gene | |||||||
| Codon 46 | Codon 89 | Codon 98 | Codon 172 | Codon 358 | Codon 426 | Codon 454 | Codon 394 | Codon 464 | |||
| CM-0237 | Susceptible | Negative | TTT | GGG | CTC | ATG | TTA | GAG | TGT | CCT | ATT |
| AF293 | Susceptible | Negative | 137TAT* | 338GGA | CTC | 585GTG* | 1145TTG | 1350AAG* | 1433TGC | CCT | ATT |
| CM-2495 | Susceptible | Negative | 137TAT* | 338GGA | CTC | 585GTG* | 1145TTG | 1350AAG* | 1433TGC | 1285CCG | 1495ATA |
| CM-2627 | Resistant | Positive | TTT | GGG | 364CAC* | ATG | TTA | GAG | TGT | 1285CCG | ATT |
| Amino acid | F46 | G89 | L98 | M172 | L358 | E426 | C454 | P394 | I464 | ||
| Substitutions* | F46Y* | L98H* | M172V* | E426K* | |||||||
Nucleotides are numbered from the translation start codon ATG of cyp51A and cyp51B. The numbers indicate the position at which a base change occurs (in bold). When a base pair change resulted in amino acid substitutions the change is shown.
Genotyping and phylogenetic analyses of the two A. fumigatus clinical isolates were also done. Comparative sequence analysis of portions of the locus AFUA_3G08990, encoding a putative cell surface protein (CSP)15,17 was performed with the two consecutive isolates and a panel of 22 A. fumigatus control strains without temporal or geographical relationships. Genotyping of single spore strains was done by PCR amplification and sequencing of the CSP polymorphic loci to establish if the two strains were identical. A. fumigatus-specific primers: 5′-TTGGGTGGCATTGTGCCAA-3′ (forward) and 5′-GGAGGAACAGTGCTGTTGGTGA-3′ (reverse) were used for PCR amplification, as previously described.15,17 These primers amplify a 550–700-bp fragment of the AFUA_3G08990 gene (dependent on the number of repeats). A phylogenetic analysis of all the CSP gene sequences was performed using InfoQuest FP software v4.50 (Bio-Rad Laboratories, Madrid, Spain). The Neosartorya fischeri NRRL 181 CSP gene sequence (GenBank accession number XM_001263541) was included as the out-group taxon in order to root the resultant trees.
The CSP analysis results indicated that the two clinical strains had unique genetic profiles, showing that both A. fumigatus strains were not related genetically. This finding was expected as results from cyp51 gene sequencing indicated differences in nucleotide sequences between both strains which proved a different phylogenetic origin and nearly discarded the possibility of a clonal origin.
The number of reports of resistance of clinical isolates of A. fumigatus to itraconazole and other azole agents is gradually increasing.19–23 Azole-resistant invasive aspergillosis, in primary or breakthrough infections, which failed to respond to itraconazole or voriconazole treatment, have been previously described.19,22 High MIC values of azole compounds and several mutations in cyp51 genes have been associated to those clinical failures.8
The L98H mutation in combination with the tandem repeat (TR) alteration was described by Mellado et al. for A. fumigatus.14 These changes were described as one of the molecular alterations in Cyp51 causing cross resistance to azole agents in A. fumigatus. In addition, this mechanism of resistance has been recently associated to environmental use of plaguicides in agriculture and gardening to prevent crop failure due to fungal plant pathogens.24,25 The mechanism of action of those plaguicides, 14-α-demethylase inhibitors (DMIs), is similar to that of azole compounds, already described in some other fungal species such as Penicillium digitatum and Blumeriella jaapii which are well-known plant pathogens.26,27
The azole resistant A. fumigatus isolate (CNM-CM-2627) harbored the L98H mutation in combination with the TR alteration. To our knowledge, this is the first case of this mutation in a clinical isolate in Spain, showing that A. fumigatus multiple azole resistance has been present in Spain since at least 2003. This resistance mechanism was described in clinical strains of A. fumigatus Dutch patients isolated in 1999. Since then, cross azole resistant strains harboring this mutation have been recovered in other countries such as Denmark, the United Kingdom and China.20,28–30 This suggests that these molds might be exposed to the selective pressure of azole compounds and other 14-α-demethylase inhibitors either in the patient or in the environment. This aspect requires further study, especially in areas where those plaguicides are extensively used.
Long-term azole therapy may increase the risk of selecting strains exhibiting reduced susceptibility to these compounds. In this case study, however, the resistant A. fumigatus isolate was genetically different from the susceptible isolate, suggesting eradication and replacement with the new strain. We can also speculate about the isolation of A. nidulans. Patient could have inhaled environmental conidia and then those with decreased susceptibility to azole were selected.
These findings suggest that in all long-term treatments with antifungal agents, especially with azoles, repeated sampling and regular susceptibility testing of isolated strains is necessary, especially in immunosuppressed patients whose infection often requires treatment for several weeks or months, as it is very difficult to predict the evolution of the development of resistance by the same strain or replacement by a new strain.
Conflict of interestIn the past 5 years, M.C.E. has received grant support from Astellas Pharma, bioMerieux, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering Plough, Soria Melguizo SA, the European Union, the ALBAN program, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, The Spanish Health Research Fund, The Instituto de Salud Carlos III, The Ramon Areces Foundation, The Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough.
In the past 5 years, J.L.R.T. has received grant support from Astellas Pharma, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering Plough, Soria Melguizo SA, the European Union, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, The Spanish Health Research Fund, The Instituto de Salud Carlos III, The Ramon Areces Foundation, The Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp and Dohme, Mycognostica, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering Plough.
EM declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
EM was supported by the Research Projects from the Spanish Ministry of Science and Innovation: SAF2008-04143 and ERA-NET Pathogenomics (7th FP), BFU2008-04709-E/BMC.