Species of phylum Basidiomycota are of great interest in many studies as a source of valuable biological compounds.
AimsTo estimate the levels of antioxidant compounds (phenolic acids, indole compounds and sterols) in edible species of Aphyllophorales (sensu lato): Hydnum repandum L. and Sparassis crispa (Wulf.) Fr.
MethodsA reversed-phase high performance liquid chromatography (RP-HPLC) method was used for the quantitative and qualitative analyses of antioxidant components (phenolic acids, indole compounds, and sterols).
ResultsAnalysis of phenolic acids revealed that 8 out of the 10 analyzed compounds were present. The quantitatively predominant in Hydnum repandum was protocatechuic acid (75.23mg/100g DW), and in Sparassis crispa it was p-hydroxybenzoic acid (43.92mg/100g DW). In turn, analysis of indole compounds identified 5 out of the 12 analyzed compounds: indole, melatonin, serotonin, tryptamine, and l-tryptophan. Moreover, ergosterol was also identified and quantitatively determined (150.37mg/100g DW) in Sparassis crispa extracts.
ConclusionsThe data presented in this paper confirm the significant potential of chemical components with recognized antioxidant activity. The species can be considered as an alternative source of phenolic acids and ergosterol.
Los hongos del filo Basidiomycota son de gran interés en diversas investigaciones por ser una fuente de compuestos biológicos valiosos.
ObjetivosDeterminar los niveles de compuestos antioxidantes (ácidos fenólicos, compuestos de indol y esteroles) en las especies comestibles de Aphyllophorales (sensu lato) Hydnum repandum L. y Sparassis crispa (Wulf.) Fr.
MétodosEl método RP-HPLC se empleó en el análisis cuantitativo y cualitativo de los siguientes componentes con actividad antioxidante: ácidos fenólicos, compuestos de indol y esteroles.
ResultadosEl análisis de los ácidos fenólicos reveló que 8 de los 10 compuestos analizados estaban presentes. El ácido cuantitativamente predominante en Hydnum repandum era el ácido protocatéquico (75,23mg/100g DW) y en Sparassis crispa el ácido p-hidroxibenzoico (43,92mg/100g DW). A su vez, el análisis de compuestos de indol identificó 5 de los 12 compuestos en estudio: indol, melatonina, serotonina, triptamina y l-triptófano. Por otra parte, en los extractos de Sparassis crispa se determinó también cuantitativamente la presencia de ergosterol (150,37mg/100g DW).
ConclusionesLos datos presentados en este trabajo confirman el gran potencial de los componentes químicos con actividad antioxidante reconocida. Las especies pueden ser consideradas como una fuente alternativa de ácidos fenólicos y ergosterol.
The role of antioxidants in the prevention of oxidative stress and associated diseases has led to the search for new, safe and natural sources of antioxidants. Edible mushrooms can be used directly in the human diet to combat oxidative stress, while inedible species represent a source of extractable phenolic compounds for use either as additives in the food industry or as components in pharmaceutical formulations because of their well-known antioxidant properties.1,2 The antioxidants found in mushrooms are mainly phenolic compounds, but there are also carotenoids, flavonoids, indole compounds, vitamins and sterols.6
The object of the present study were two wild edible mushrooms: Hydnum repandum L. (Hydnaceae) and Sparassis crispa (Wulf.) Fr. (Sparassidaceae) commonly growing in Polish forests. These species belong to the artificial taxon known as Aphyllophorales.
The aim of the study was to determine the levels of antioxidant compounds in extracts of fruiting bodies. This study is a continuation of an extensive analysis of the chemical composition of mushrooms to confirm their usefulness as potential sources of compounds with antioxidant properties.13,15,19 As far as we know, our study is the first where sterols and indole compounds have been described in the examined fungi species.
Materials and methodsFungal materialsSamples of mushrooms were collected in 2008–2010 in mixed forests of northern Poland. The mature fruiting bodies were lyophilized at temperature −40°C. Taxonomic identification was conducted according to Gumińska and Wojewoda.7 Representative voucher samples are deposited in the Department of Pharmaceutical Botany UJCM, Kraków, Poland.
Determination of phenolic acidsFive grams of powdered mushroom material were hydrolysed with 2M hydrochloric acid for 2h at 100°C. Hydrolysates were extracted with 50ml of ethyl acetate and concentrated to dryness in a rotary vacuum evaporator (Büchi, Germany) at 40°C. The HPLC method was performed according to Ellnain-Wojtaszek and Zgórka.5 HPLC analyses were conducted using an HPLC VWR Hitachi-Merck apparatus: autosampler L-2200, pump L-2130, LiChrospher RP-18e column (250mm×4mm, 5μm) thermostated at 25°C, column oven L-2350, diode array detector L-2455 at UV range 200–400nm. The mobile phase consisted of solvent A: methanol/0.5% acetic acid 1:4 (v/v), and solvent B: methanol. The gradient was as follows: 100:0 for 0–25min; 70:30 for 35min; 50:50 for 45min; 0:100 for 50–55min; 100:0 for 57–67min. Phenolic acid standards of ferulic, p-hydroxybenzoic, and vanillic acids were from Fluka (Chemie AG), and those of caffeic, chlorogenic, cinnamic, o-coumaric, protocatechuic, sinapic, and syringic acids were from Sigma (St. Louis, USA). The quantitative analysis of phenolic acids was performed with the use of a calibration curve with the assumption of the linear size of the area under the peak and the concentration of the reference standard. Three calibration curves for each reference standard were prepared using the following concentrations: 0.5, 0.125, 0.0625, 0.03125, 0.015625mg/ml.
Determination of indole derivativesFive grams of powdered mushroom material were extracted with 100ml of methanol for 2h on a magnetic stirrer at 22±2°C. Mixed extracts (500ml) were concentrated to dryness using a rotary vacuum evaporator at 22±2°C. The HPLC method was performed according to the procedure described by Muszyńska.15 HPLC analyses were conducted using a Hitachi apparatus with an L-7100 pump, L-7400 UV Detector (λ=280nm) and Purospher RP-18e column (250mm×4mm, 5μm) thermostated at 25°C. The mobile phase was composed of methanol/water/ammonium acetate 15:14:1 (v/v/v). Indole standards: l-tryptophan, 5-hydroxytryptophan, 5-methyltryptophan, serotonin, melatonin, tryptamine, 5-methyltryptamine, kynurenine, indole-3-acetic acid, 3-indoleacetonitrile, indole and indole-acetamide were from Sigma-Aldrich (Germany).
Determination of sterolsFive grams of each powdered mushroom were mixed with 100ml of a 75:25 (v/v) mixture of methanol and dichloromethane, followed by sonication for 10min and centrifugation at 10000rpm for 5min. Merged extracts (300ml) were concentrated to dryness using a rotary vacuum evaporator at 22±2°C. The HPLC method was performed according to the procedure developed by Yuan.20 The mobile phase consisted of solvent A: methanol/water 20:80 (v/v), and solvent B: methanol/dichloromethane 75:25 (v/v). A gradient procedure was used as follows: starting at sample injection, 60% of B for 5min; a linear gradient from 60 to 100% of B for 10min; and 100% of B for 10min. The flow rate was 1.0ml/min. The chromatographic peaks were recorded at a wavelength of 280nm to facilitate the detection of ergosterol and 266nm for ergocalciferol. Sterol standards, ergosterol, and ergocalciferol were obtained from Fluka. The quantitative analysis of ergosterol was performed with the use of a calibration curve with the assumption of the linear size of the area under the peak and the concentration of the reference standard. Three calibration curves for each reference standard were prepared using the following concentrations: 0.5, 0.125, 0.0625, 0.03125, 0.015625mg/ml.
Statistical analysisAll the analyses were performed in triplicate and the results were presented as mean values with standard deviations. The differences in appropriate chemical compound contents between species were compared by ANOVA at significant level p<0.05.
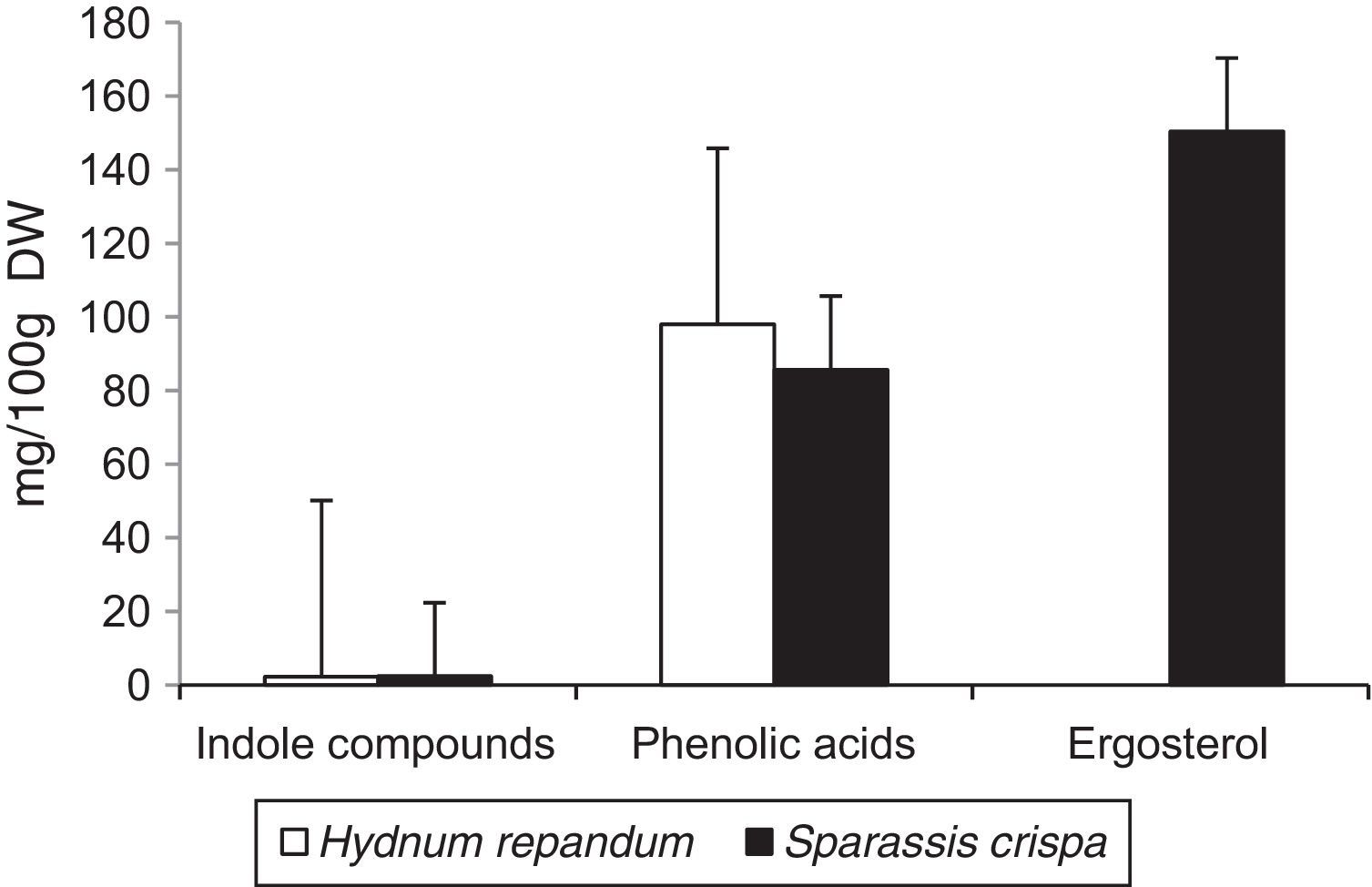
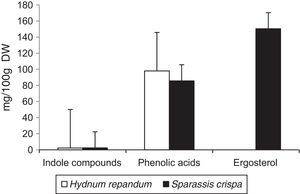
Results and discussionThe results of the performed analyses are shown in Table 1. Among the 10 studied phenolic acids, five of them were detected in the extracts from the fruiting body of H. repandum; additionally, cinnamic acid was identified. However, seven phenolic acids were found in similar extracts of S. crispa. The total amounts of phenolic acids and cinnamic acid were 97.96 and 85.65mg/100g DW in H. repandum and S. crispa, respectively (Fig. 1).
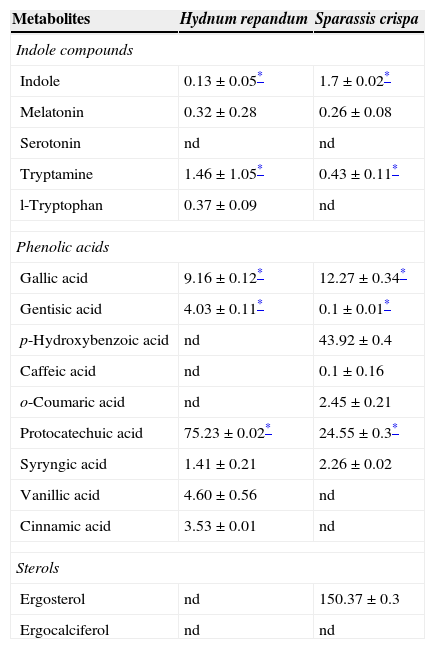
Amounts (mg/100g DW) of antioxidant compounds in extracts of Hydnum repandum and Sparassis crispa species.
| Metabolites | Hydnum repandum | Sparassis crispa |
|---|---|---|
| Indole compounds | ||
| Indole | 0.13±0.05* | 1.7±0.02* |
| Melatonin | 0.32±0.28 | 0.26±0.08 |
| Serotonin | nd | nd |
| Tryptamine | 1.46±1.05* | 0.43±0.11* |
| l-Tryptophan | 0.37±0.09 | nd |
| Phenolic acids | ||
| Gallic acid | 9.16±0.12* | 12.27±0.34* |
| Gentisic acid | 4.03±0.11* | 0.1±0.01* |
| p-Hydroxybenzoic acid | nd | 43.92±0.4 |
| Caffeic acid | nd | 0.1±0.16 |
| o-Coumaric acid | nd | 2.45±0.21 |
| Protocatechuic acid | 75.23±0.02* | 24.55±0.3* |
| Syryngic acid | 1.41±0.21 | 2.26±0.02 |
| Vanillic acid | 4.60±0.56 | nd |
| Cinnamic acid | 3.53±0.01 | nd |
| Sterols | ||
| Ergosterol | nd | 150.37±0.3 |
| Ergocalciferol | nd | nd |
Values are means of three experiments±SD.
nd – not detected.
The analysis of phenolic acids presented here is a supplementation of earlier studies conducted by Barros et al.4 Phenolic acids derived from benzoic and cinnamic acids (7.40mg/100g DW) were detected in extracts from the fruiting bodies of mushrooms collected in Portugal. In the fruiting bodies of H. repandum, gallic acid predominated (4.17mg/100g DW).4 Our studies on H. repandum have proved the presence of the above-mentioned phenolic acids, i.e., gallic and protocatechuic (the highest levels being 75.23mg/100g DW), as well as cinnamic acid. Additionally other phenolic acids, not mentioned by Puttaraju, were detected: i.e. gentisic, syringic, and vanillic acids.18 Previous chemical analyses of S. crispa had shown the presence of numerous phenolic acids in the fruiting bodies, with the highest levels of benzoic acid (0.348mg/100g DW).10,18 In our studies of S. crispa extracts, the presence of the following acids was confirmed: gallic, gentisic, p-hydroxybenzoic, caffeic, o-coumaric, protocatechuic and syringic acids; p-hydroxybenzoic acid was the dominant one (43.92mg/100g DW). Qualitative and quantitative differences in the levels of phenolic compounds in mushroom fruiting bodies might be the result of dissimilar genetic parameters, specific sites where they were acquired, variations in environmental conditions during growth and maturation, and air pollution.
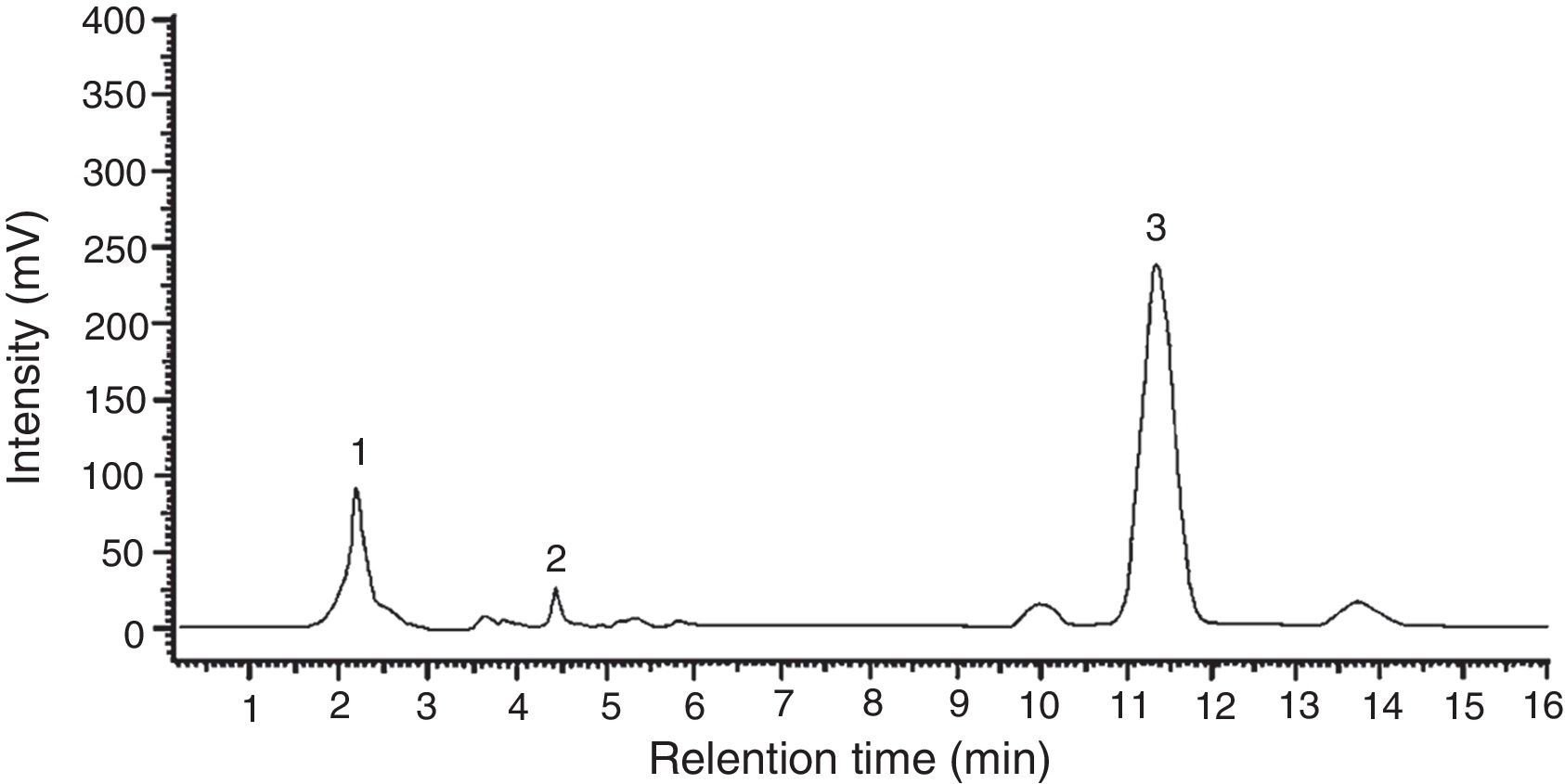
Among the 12 indole compounds analyzed, four of them were detected in the extracts from H. repandum as well as in those of S. crispa; i.e. indole, melatonin, tryptamine and l-tryptophan. In addition, trace amounts of serotonin were detected in H. repandum. The total amounts of indole compounds were similar (2.28 and 2.39mg/100g DW in H. repandum and S. crispa extracts, respectively) (Fig. 1). A representative chromatogram is presented in Fig. 2.
Our previous study concerned on the concentrations of non-hallucinogenic indole compounds in the fruiting bodies of edible mushrooms. In the methanolic extracts of Armillaria mellea, Agaricus bisporus, Boletus edulis, Boletus badius, Cantharellus cibarius, Lactarius deliciosus, Leccinum rufum, Pleurotus ostreatus, Suillus luteus, and Tricholoma equestre, the amounts of indole compounds ranged from 0.01 to 34.11mg/100g DW. Serotonin, tryptamine and methyltryptamine were present in the highest quantities.14–16 The present study is the first one in which indole compounds in H. repandum and S. crispa have been examined, and the results show much lower quantities of the components previously studied in edible species.17 Only tryptamine in H. repandum and indole in S. crispa were at certain levels that suggested that these mushrooms might be a potential source of these medicinally important compounds. Tryptamine is found in trace amounts in the brains of mammals and is believed to play a role as a neuromodulator or neurotransmitter.8,9,11
Ergosterol was the only representative of the analyzed compounds with a sterol structure, and was detected in high amount in S. crispa extracts (150.37mg/100g DW) (Fig. 1). It is the main constituent of mushroom cell membranes. The highest levels of ergosterol have been noted in saprophytic fungi and may constitute up to 83–89% of the entire amount of sterols.
Studies on the sterol concentration in certain higher mushrooms have shown that the overall sterol levels ranged from 625 to 774mg/100g DW. To compare, the average ergosterol level in genus Lactarius is 296–300mg/100g DW, and in Cantarellus 304–377mg/100g DW.12
Other studies have shown the occurrence of ergosterol in numerous wild-grown and cultivated edible mushrooms belonging to Ascomycota and Basidiomycota.3 Quantitative analysis with HPLC has confirmed the presence of ergosterol (55–352mg/100g DW) in fruiting bodies and also proved that ergosterol is accumulated in significant amounts. Our research has shown that S. crispa is an example of fungi with a significant ergosterol content in fruiting bodies.
ConclusionsThe wild mushrooms studied in this project contain important antioxidant molecules that could play a protective role in diseases related to oxidative stress, such as cancer and cardiovascular diseases and can be considered as potential sources of phenolic acids and ergosterol.
Conflict of interestThe authors declare they have no conflict of interest.