The correlation between sagittal balance of the spine and clinical outcome after vertebroplasty (VP) in patients with osteoporotic vertebral compression fractures (OVCF) is poorly investigated. We analysed the clinical outcome of patients with OVCF undergoing VP taking into account sagittal balance.
MethodsThe primary endpoint was the change in axial back pain; disability and health-related quality of life using VAS, ODI and SF-36 respectively in correlation to the parameters that define sagittal balance (SVA). Radiographic assessment included full spine standing lateral films. Imaging and clinical data were collected pre and post procedure at 1, 3 and 12 months.
ResultsFifty-one patients were included presenting a total of 113 OVCF. Thirty patients (60.7%) had multiple OVCF. Comparing the evolution of VAS and ODI throughout the follow-up it does not seem that there are significant differences in their behaviour between the SVA>50mm and the SVA<50mm groups (p>0.05). On the contrary, preVP SF-36 scores showed worst results in the SVA>50mm group in the physical functioning section (PF) (p<0.05) and in the physical component score (PCS) (p<0.05). These differences were maintained until 3 months of follow-up in the case of the PCS and until the end of follow-up in the case of the PF (p<0.05).
ConclusionsPatients with a SVA>50mm showed a slower recovery of their quality of life after VP for OVCF, but without significant differences with respect to pain or disability, when compared with patients with SVA<50mm.
La correlación entre el equilibrio sagital de la columna y el resultado clínico tras una vertebroplastia (VP) en pacientes con fractura vertebral osteoporótica por compresión (FVOC) ha sido poco estudiado. Analizamos el resultado clínico de la VP en pacientes con FVOC teniendo en cuenta el equilibrio sagital.
MétodosEl objetivo primario es valorar el cambio en el dolor axial, la discapacidad y la calidad de vida relacionada con la salud mediante la escala analógica visual (VAS), índice de discapacidad de Oswestry y el test SF-36 respectivamente. Todo ello, correlacionado con el eje sagital vertical (SVA) que define el equilibrio sagital. El estudio radiográfico consistió en una radiografía de perfil de columna completa en bipedestación. Los controles clínico-radiológicos se realizaron pre- y pos-VP (1,3 y 12 meses).
ResultadosSe incluyeron 51 pacientes con un total de 113 FVOC. Treinta pacientes (60,7%) presentaron múltiples FVOC. Comparando la evolución del resultado del VAS y del índice de discapacidad de Oswestry durante el seguimiento no se observaron diferencias significativas entre los grupos de pacientes con SVA <50mm y >50mm (p>0,05). Por el contrario, pre-VP los resultados del SF-36 presentaba peores puntuaciones en el grupo de SVA >50mm en la sección de función física (p<0,05) y de componente de salud física (p<0,05). Estas diferencias se mantenían hasta los 3 meses de seguimientos en componente de salud física y hasta el final del seguimiento en la sección de FP (p<0,05).
ConclusionesLos pacientes con SVA >50mm presentan una recuperación más lenta de su calidad de vida relacionada con la salud tras VP por FVOC, pero sin diferencias significativas con respecto al dolor o discapacidad cuando se comparan con pacientes con SVA <50mm.
Although vertebroplasty (VP) is commonly used in patients with osteoporotic vertebral compression fractures (OVCF), the clinical and radiological factors that influence outcome are unclear1-3. The aim of this study was to investigate whether the presence of sagittal imbalance influences clinical outcome after VP from the point of view of health-related quality of life, disability and/or pain with the intention of identifying a new prognostic factor, sagittal imbalance, that would allow us to recognise a group of patients who may be predicted to have a worse final outcome after VP for OVCF.
Material and methodsOnce approval had been obtained from the Ethics Committee, those patients in our centre with OVCF who underwent VP were studied over a period of 24 months.
OVCF was defined as a bone fragility fracture secondary to a low energy mechanism or with a bone densitometry (BMD) T-score less than or equal to −2.5. OVCFs were diagnosed in all cases by clinical assessment, plain radiology and magnetic resonance imaging (NMRI) or SPECT-CT in cases where NMRI could not be performed.
All fractures that were not osteoporotic (metastasis, infection, etc.) or those with neurological symptoms were excluded from the study.
The indication for VP was established for symptomatic OVCF (>5 on the visual analogue scale [VAS>5]) that did not improve after 6 weeks of conservative treatment. No maximum time limit was established for the evolution of the fracture since, in our experience, OVCFs can be symptomatic for a long period of evolution, especially if they are complicated by vertebral avascular necrosis. On the other hand, patients were sometimes referred to our hospital with a fracture evolution time of more than 6 weeks.
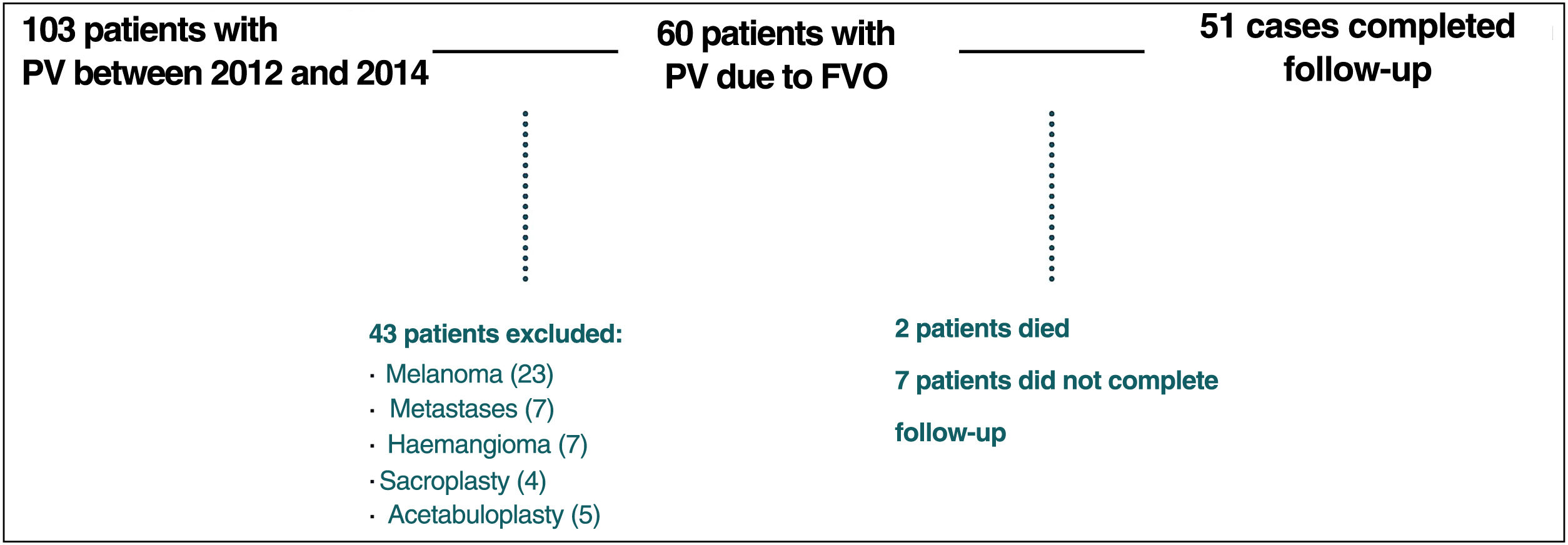
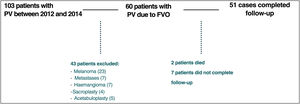
Of the 60 patients initially included, 9 patients were excluded: 2 patients died during the first 6 months of follow-up due to causes external to the study, while 7 patients did not complete the follow-up protocol (Fig. 1).
Assessment of clinical outcomes was performed by analysing pain, health-related quality of life (HRQoL) and disability using standardised questionnaires: the visual analogue scale (VAS), Oswestry disability index (ODI) and the SF-36 test (SF-36) before and after the procedure (1, 3 and 12 months).
Morbidity was previously analysed using the Charlson comorbidity index (CCI) and the American Society of Anesthesiologists (ASA) anaesthesia risk scale.
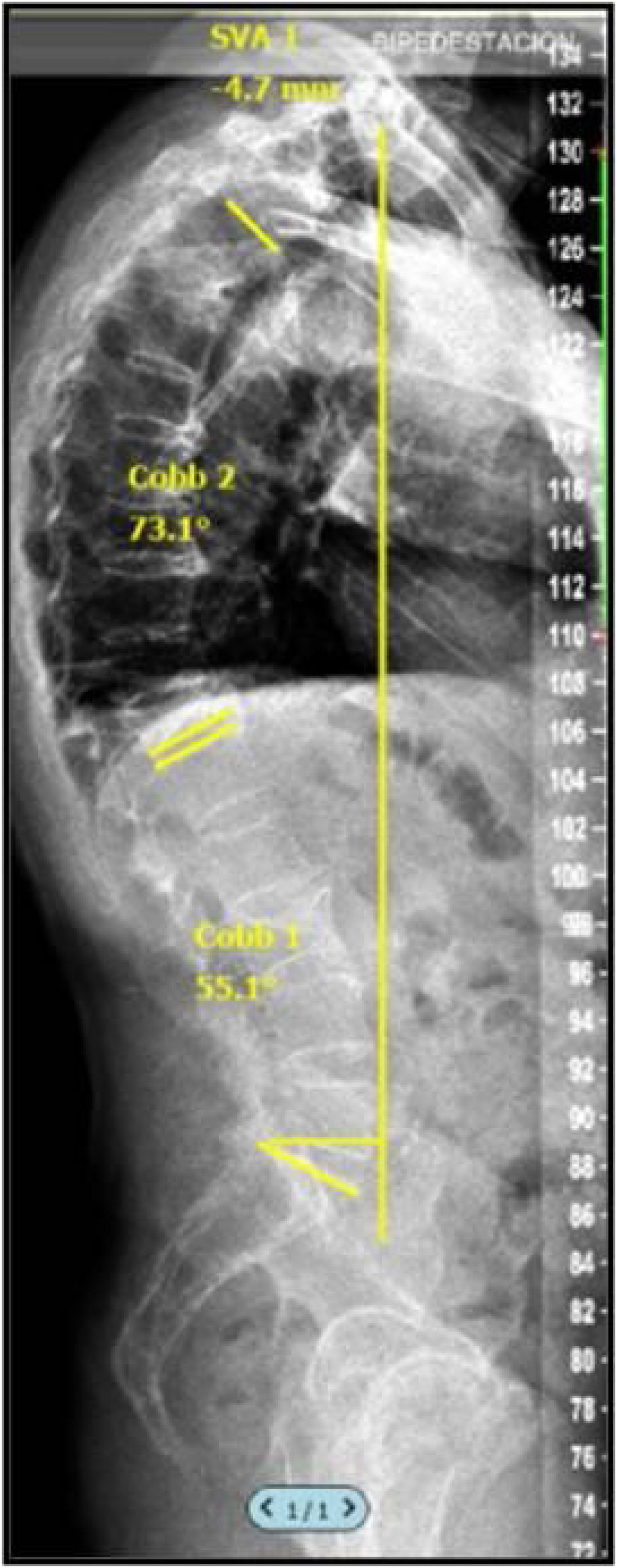
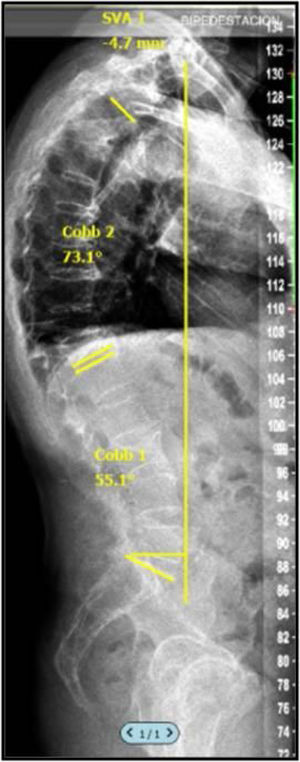
Radiographic assessment was performed by analysing full spine radiographs (anteroposterior and lateral) in clavicle position including C7 and femoral heads before the procedure and at 1, 3 and 12 months post-PV allowing characterisation of sagittal balance by calculating the sagittal vertical axis (SVA) (Fig. 2). The VAS value can be influenced by the position of the patient's arms, and therefore it is very important that all radiographs are obtained with a standardised patient position. In the study by Horton et al.,4 where they describe the “clavicle position” (patient in a standing position with hips and knees extended, arms flexed to 30 with elbows and wrists flexed and hands in a relaxed fist placed in the supraclavicular fossa without external help), they state that this is the best position to visualise the landmarks on a full spine profile radiograph to calculate SVA.
In line with previous studies,5 the current sample was divided into SVA > or <50mm, following the recommendations of other authors who established an SVA of 50mm as the limit of normality. The digital images obtained were evaluated and compiled in a PACS system with DICOM format, so that the lead author could perform the sagittal balance study with SURGIMAP® (Nemaris Inc., New York, NY, USA), a programme validated for this purpose.6,7
Vertebroplasty procedureAll procedures were performed in an angiography room of the radiology department under scopic control or in the computed tomography (CT) room. The procedure was performed with the patient fasting for 6h and in prone decubitus under sterile conditions and with prophylactic administration of antibiotics 1h before.
After pedicle identification, with the patient under general anaesthesia or intravenous sedation, the subcutaneous cellular tissue and periosteum were infiltrated with local anaesthetic (lidocaine 2%). Once the pedicle was located, 11-gauge needles 10–15cm long (Optimed, Ettlingen, Germany) were placed transpedicularly or extrapedicularly depending on the location of the vertebra to be treated. Subsequently, polymethylmethacrylate (PMMA) cement (Kyphon HV-R, Medtronic Sofamor Danek, Memphis, TN, USA) was injected with the reusable injection system PM-Cement (Optimed, Ettlingen, Germany). After completion of the injection, the needle was removed and haemostasis was performed. The contralateral hemivertebra was treated in the same way when a bilateral injection technique was used. Most patients were discharged within 24h after the procedure.
Statistical analysisContinuous variables have been described by mean with 95% confidence intervals (95% CI) and absolute range (minimum, maximum), while categorical variables have been described by absolute frequencies and percentages. The clinical evolution of patients was assessed using generalised estimating equation (GEE) models with an autoregressive matrix of order 1 (AR (1)) to account for intra-individual correlations.
Given the observational and exploratory characteristics of the study, the sample size was set by feasibility. Clinical activity data were systematically retrieved during the study. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25.0 (IBM Corp, Armonk, NY) with a bilateral type I error of 0.05.
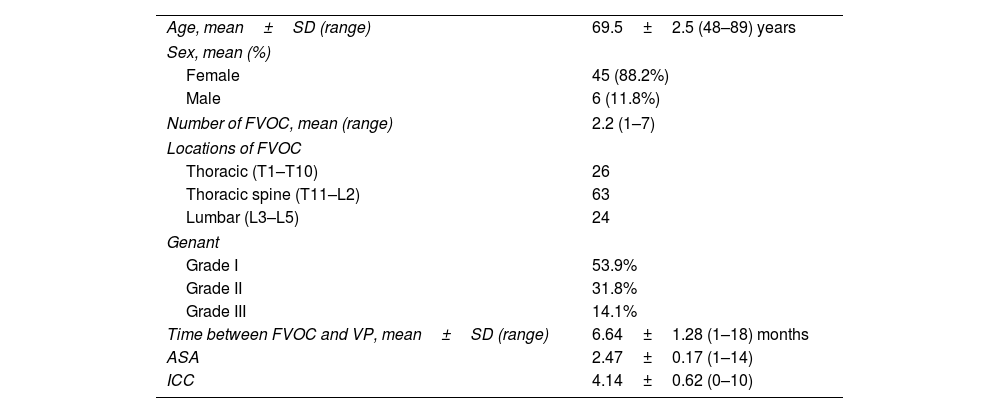
ResultsSampleFifty-one patients, 6 men (11.8%) and 45 women (88.2%), with a mean age of 69.5 years were included (95% CI: 67, 72) (range: 49–89 years). These 51 patients had a total of 113 OVCFs with a mean of 2.21 OVCFs per patient (range: 1–7). A total of 30 patients (58.8%) had multiple OVCFs. The vertebral levels treated ranged from T5 to L5, with the thoracolumbar junction levels, more specifically L1 (22 VP), T12 (18 VP) and T11 (14 VP), being the most frequently treated.
The mean time from symptom onset to VP was 6.64 months (95% CI: 5.36, 7.91) (range: 1.5–18 months). Of the 113 fractures, 61 (53.9%) had a mild vertebral deformity with a maximum height loss in the anterior, middle or posterior portion of the vertebral body of 20–25% (Genant 1). 31.8% (36 vertebrae) had a maximum height loss of 25–40% (Genant 2), while the remaining 14.1% (16 vertebrae) had a height loss of more than 40% (Genant 3).
In terms of wedging of affected vertebrae, there was no significant difference between pre-PV and 12 months post-PV, with an average change of .32 (95% CI −0.12, 0.76) (p=0.151).
Regarding the pre-procedural morbidity of the studied sample, the mean Charlson comorbidity index (CCI) value was 4.14 (95% CI: 3.52, 4.76) (range: 0–10) and the mean ASA value was 2.47 (95% CI: 2.3, 2.64) (range: 1–4). During follow-up, only 3 cases of new fractures (either refracture of the operated vertebra or fracture distal to it) were recorded among the 113 procedures performed, representing an incidence of 2.6%. The baseline characteristics of the patients who completed follow-up at 12 months are shown in Table 1.
Baseline characteristics of patients who finished follow-up after 12 months.
| Age, mean±SD (range) | 69.5±2.5 (48–89) years |
| Sex, mean (%) | |
| Female | 45 (88.2%) |
| Male | 6 (11.8%) |
| Number of FVOC, mean (range) | 2.2 (1–7) |
| Locations of FVOC | |
| Thoracic (T1–T10) | 26 |
| Thoracic spine (T11–L2) | 63 |
| Lumbar (L3–L5) | 24 |
| Genant | |
| Grade I | 53.9% |
| Grade II | 31.8% |
| Grade III | 14.1% |
| Time between FVOC and VP, mean±SD (range) | 6.64±1.28 (1–18) months |
| ASA | 2.47±0.17 (1–14) |
| ICC | 4.14±0.62 (0–10) |
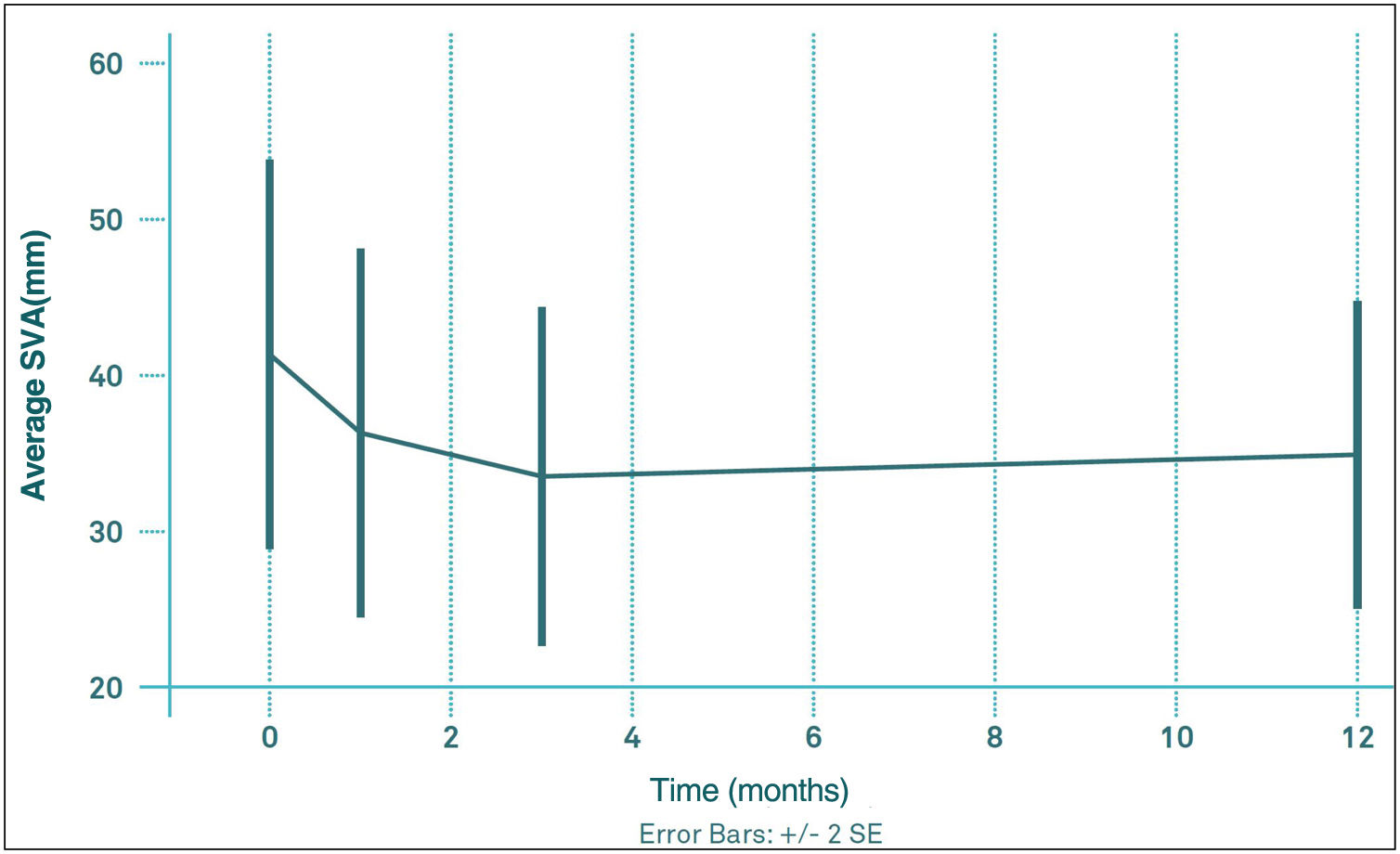
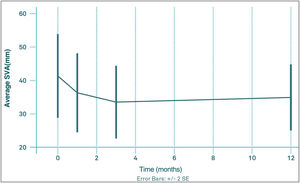
The mean values of thoracic kyphosis (TK) and lumbar lordosis (LL) before VP were 52.6 (95% CI: 49.1, 56.2) and 55.1 (95% CI: 51.2, 59.0), respectively. The mean pre-PV VAS value was 41.6mm (95% CI: 28.7, 54.5); 1 month after VP was 36.4mm (95% CI: 24.5, 48.4); 3 months after VP was 33.7mm (95% CI: 22.8, 44.7) and at 12 months was 35.0mm (95% CI: 25.0, 45.1). At no time point were these changes in VAS significant with respect to pre-PV or among any of the post-PV controls (Fig. 3). The percentage of patients with an imbalance greater than 50mm did not change significantly during the follow-up period, ranging from 26.8 to 38.2%.
Regarding lumbopelvic values, the mean value of pelvic tilt (PT) pre-PV was 20.4 (95% CI: 17.6, 23.2), the mean value of pelvic incidence (PI) pre-PV was 54.4 (95% CI: 51.1, 57.7), while the mean value of sacral slope (SS) pre-PV was 34.8 (95% CI: 31.7, 37.9).
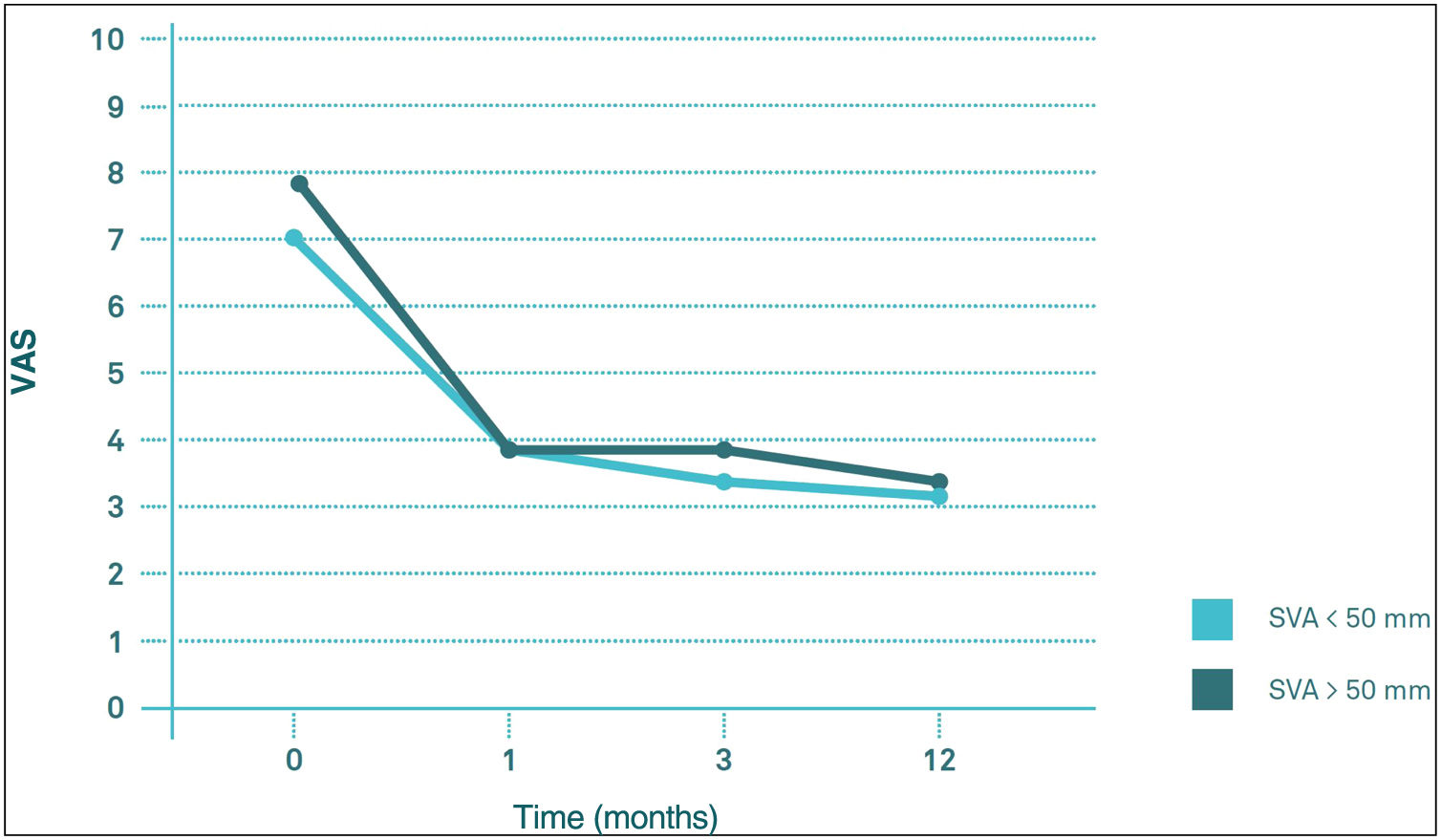
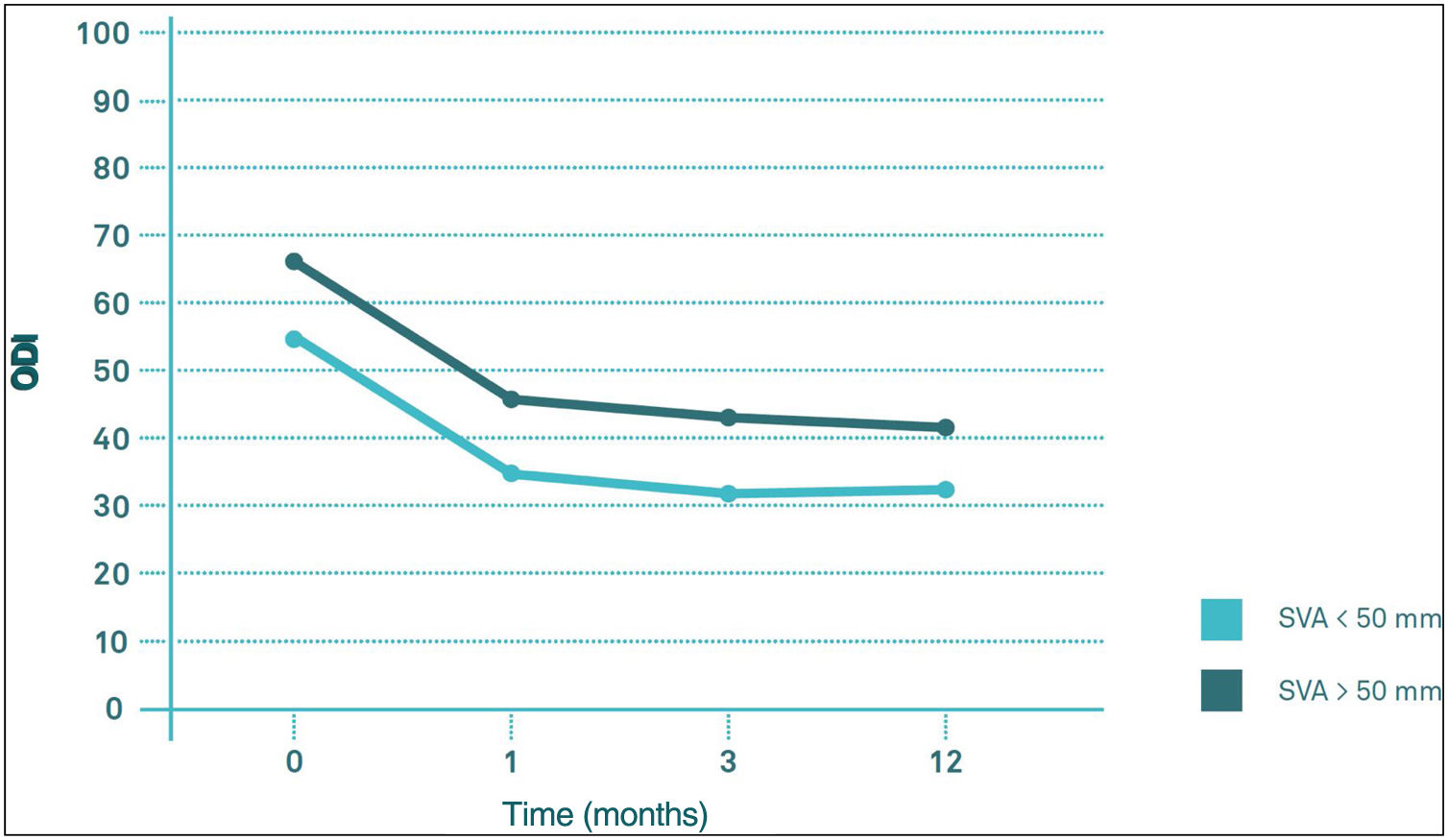
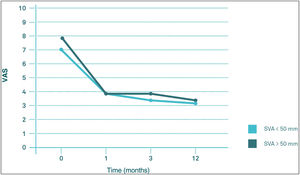
Influence of the sagittal balance on clinical outcomesComparing the evolution of VAS and ODI scores throughout follow-up, there seems to be no significant differences in their behaviour between the VAS>50mm group and the VAS<50mm group (p>0.05). The two groups start from similar pre-PV values, both experiencing a significant decrease (p<0.05) in the first month post-PV, after which they remained virtually unchanged at 3 and 12 months post-PV (Figs. 4 and 5).
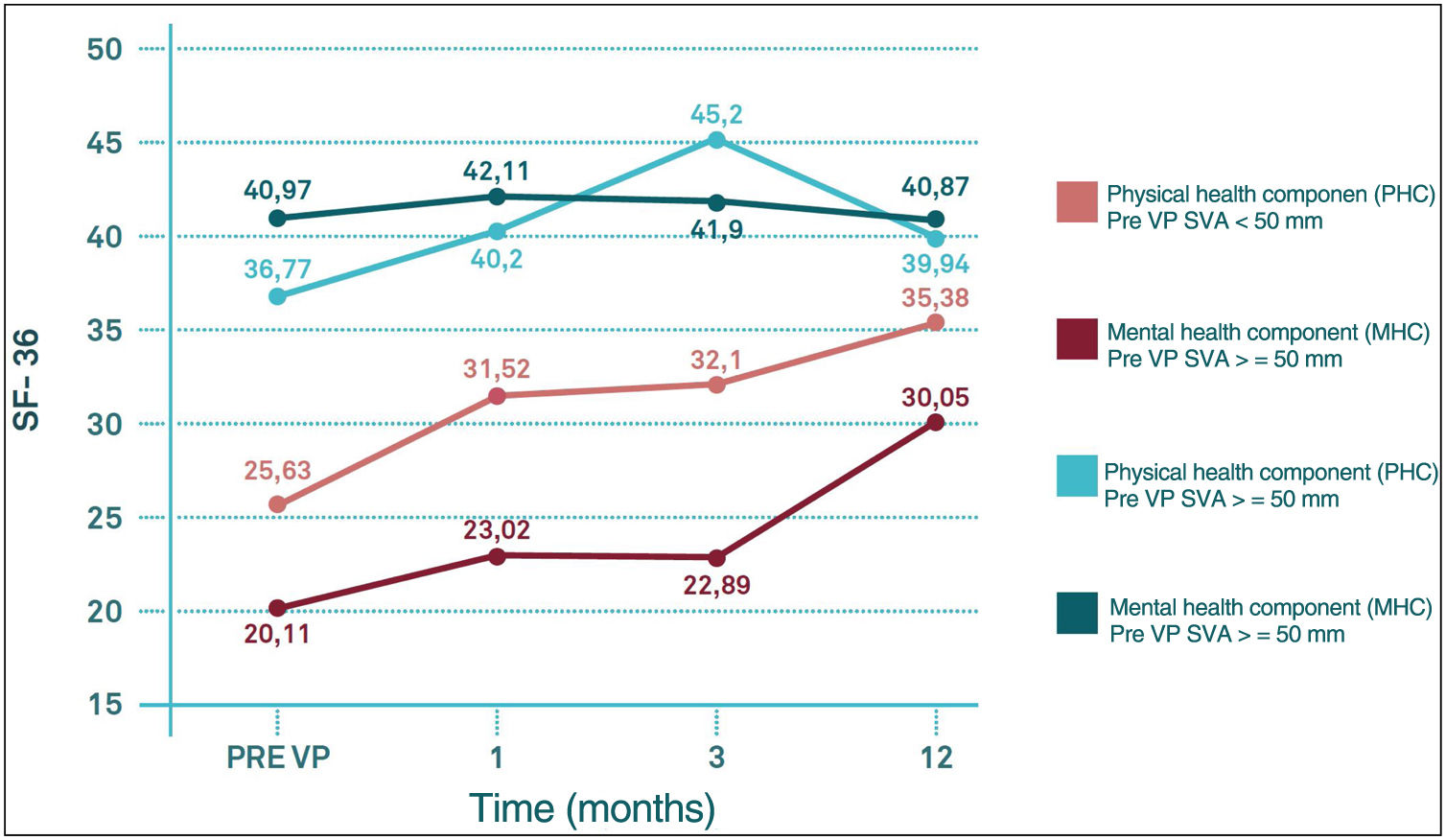
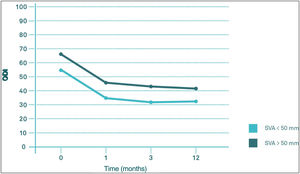
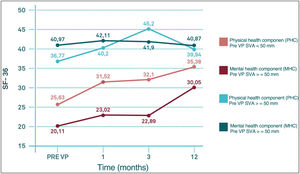
Regarding differences in the different sections of the SF-36 during follow-up, significant pre-PV differences were only observed in the physical functioning (PF) section (degree to which health limits physical activities such as self-care, walking, bending, carrying weights, and moderate and vigorous exertion), carrying weights, and moderate and strenuous exertion (p<0.05) and in the physical health component score (PHC), which summarises PF, role limitations due to physical health problems (PR), pain (PA) and general health (GH) (p<0.05), with worse results in the group with VAS>50mm. These differences were maintained until the third month of follow-up in the case of CSF, but in the case of PF they were maintained until the end of follow-up (p<0.05). In the other sections of the SF-36, there were no significant differences between the SVA>50mm and SVA<50mm groups (Fig. 6).
DiscussionAlthough most of the literature supports the effectiveness of VP in improving pain and function in patients with COVF1–3,8–15 it is still unclear which theoretical patient population is most likely to benefit from VP. The purpose of this study was to analyse the influence of sagittal balance on the outcome of VP after OVCF.
According to Glassman et al.16 in 2005 it was accepted that sagittal balance is the most important radiographic predictor of pain, disability and health status in adults with spinal deformities. However, in the field of VP in patients with OVCF only one study published in 2016 by Kim et al.17 analysed the influence of SVA on the outcome of VP in terms of pain using the VAS scale in a 12-week follow-up study in patients with acute osteoporotic single-level vertebral compression fractures. That study concluded that the outcome of VP in terms of pain is worse in patients with SVA>50mm, without analysing the influence of SVA on disability or health-related quality of life.
In contrast to the work of Kim et al., probably due to a longer follow-up period, in our series SVA does not seem to have a significant influence on either VAS or ODI. On the contrary, SVA influences SF-36 scores, with worse results in the SVA>50mm group, even before the performance of VP in the PF section (p<0.05) and in the physical summary component (PSC) score (p<0.05). These differences were maintained until the third month of follow-up in the case of the PSC and until the end of follow-up in the case of the FP section.
Therefore, it can be said that patients with a VAS>50mm presented slower recovery of PCS and restrictions in physical activities such as self-care, walking, bending, carrying weights and moderate and heavy exertion (which are the activities that define the PF section) at one year follow-up with respect to those patients with a SVA<50mm. In other words, those patients with a SVA>50mm needed more time to recover their health status such that the PHC score at 12 months post-PV in the SVA>50mm group is comparable to the PHC score at 1 month post-PV in the SVA<50mm group, while there seems to be no clear influence of VAS on the evolution of the mental summary component score (MSC).
With respect to other factors in the sample that may act as confounding factors, neither Charlson nor ASA scores were found to influence clinical outcome in patients with VAS>50mm, in contrast to the findings of Álvarez et al.8 who, like other authors, describe worse outcomes in patients with functional limitations or comorbidities.
In short, and according to the results, we could identify a new prognostic factor, sagittal imbalance, allowing us to categorise a subgroup of patients in whom we can anticipate a slower recovery after undergoing VP for OVCF, thus being able to provide these patients with more realistic expectations after undergoing VP.
Study strengths and limitationsWe consider that the present study has several limitations. Firstly, it is a prospective observational study, so we cannot rule out the biases inherent to this type of study. In addition, the number of patients included in the study is perhaps small. A larger sample size would probably have yielded different results, so the results of this single-centre study cannot be extrapolated to other centres. The authors believe that a multicentre study would be appropriate to confirm the findings of the present study.
ConclusionsPatients with SVA>50mm have a slower recovery of their health-related quality of life after VP due to OVCF, but no significant differences with respect to pain or disability when compared to patients with SVA<50mm.
Level of evidenceLevel of evidence III.
FundingThis research did not receive any specific grants from public sector agencies, the commercial sector or not-for-profit entities.
Conflict of interestsThe authors have no conflict of interests to declare.