Neurological compression occurs in 10%–20% of patients who develop spinal metastases. In the last decade, the evolution of oncological diagnostic and medical techniques, the change from conventional external radiation to radiosurgery and the new surgical instruments have meant that the treatment of these patients must be indicated in a personalized manner and by consensus, multidisciplinary way, in specific commissions.
Today, the biological state of the patient, the presence of mechanical instability, the neurological assessment and degree of epidural compression, as well as the best prognostic categorization of the tumor, are established as decision factors prior to the indication of surgical treatment, treatment that has passed from a cytoreductive concept to that of a spinal cord release from tumor in order to ensure safe radiosurgery.
La compresión neurológica se presenta entre el 10-20% de los pacientes que desarrollan una metástasis vertebral. En la última década, la evolución de las técnicas diagnósticas y médicas oncológicas, el cambio de la radiación convencional externa a la radiocirugía y los nuevos instrumentales quirúrgicos, han hecho que el tratamiento de estos pacientes deba de ser indicado de forma personalizada y en consenso, de forma multidisciplinar, en comisiones específicas.
Hoy, el estado biológico del paciente, la presencia de inestabilidad mecánica, la valoración neurológica y el grado de compresión epidural, así como la mejor categorización pronóstica del tumor, se establecen como los factores de decisión previa a la indicación del tratamiento quirúrgico, tratamiento que ha pasado de un concepto «citorreductor» al de «separador» o «preparador» de la médula para asegurar una radiocirugía segura.
In 2018 figures from the USA, 1.7 million new cancer diagnoses are made per year, of which 40%–70% will develop vertebral metastases (mx), the most frequent to develop being breast (40%), lung (15%), prostate (10%) and renal (10%) cancers. Among these, around 20% will progress to spinal cord compression. In a not insignificant number of them, the presence of clinical symptoms, manifested as pain or neurological symptoms, will involve surgical treatment. Not so long ago Patchell et al.1 demonstrated surgery's superiority over radiotherapy alone, in terms of improved neurological results, better quality of life and lower economic and social cost.
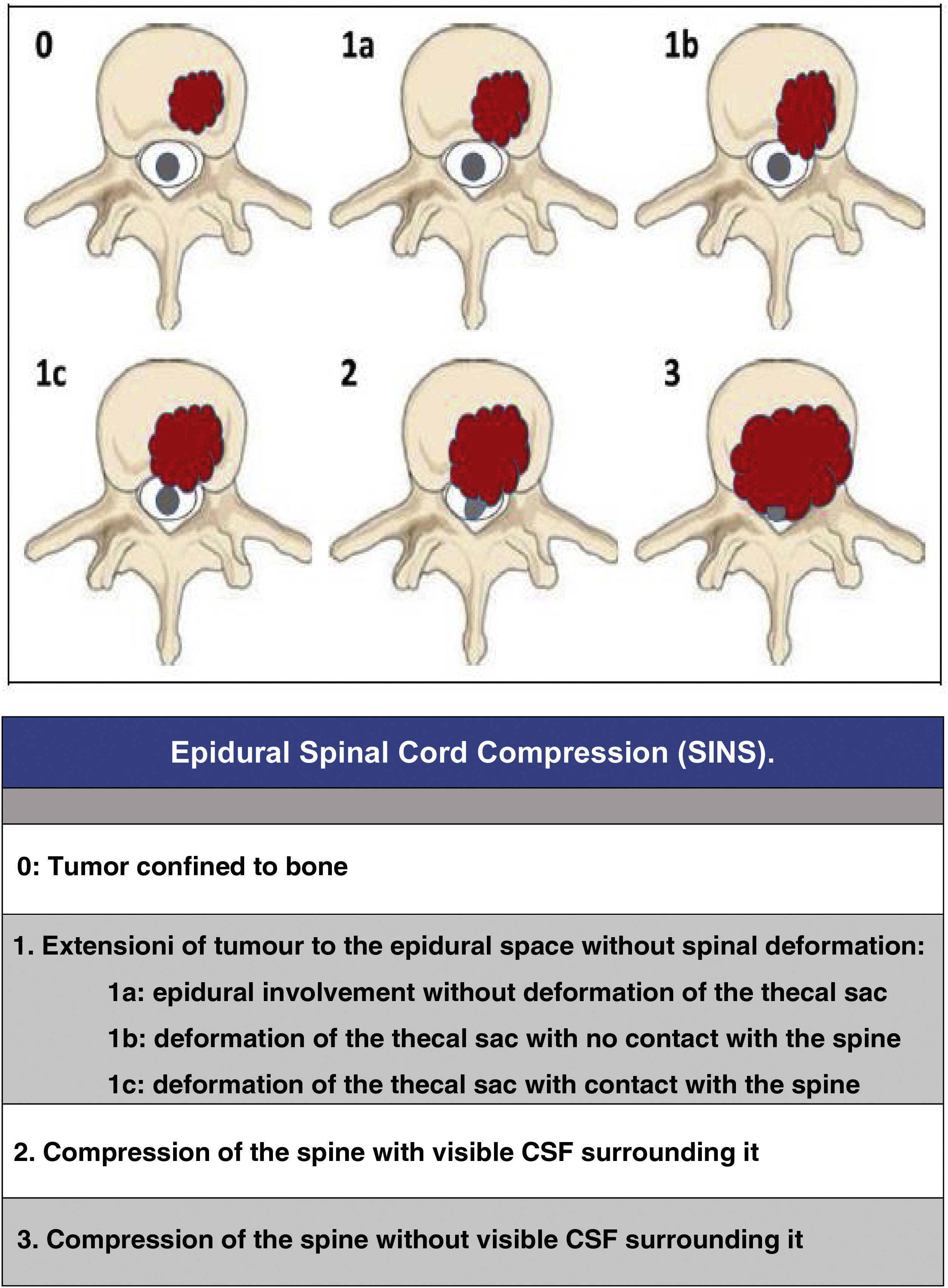
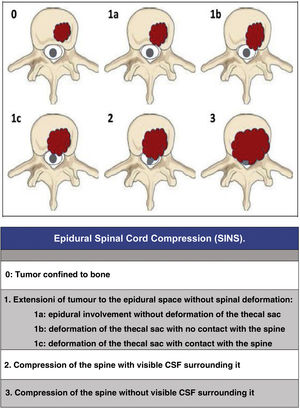
Since then, the change in the approach to the treatment of these patients has progressed beyond a single vision; the approach must be made by evaluating multiple parameters: quality of life, neurological status, primary tumour, extension, stability, etc., in order to adapt the treatment to the clinical characteristics of each patient. In this regard, we would like to highlight three now classic studies that have enabled a multidisciplinary consensus categorisation to be made, and thus achieve a better treatment adaptation: the revised scale of Tokuhashi et al., 20052 (Table 1), for the assessment of tumour disease and its metastatic burden, the Spinal Instability Neoplastic Score (SINS) 2010 for biomechanical assessment3 (Table 2) and the Epidural Spinal Cord Compression (ESCC) scale of Bilsky et al., 20104 (Fig. 1) for the assessment of the possibility of spinal cord risk in radiotherapeutic treatment. The integration of these concepts has given rise to the current approach to the treatment of metastatic pathology, the NOMS algorithm (Neurologic, Oncologic, Mechanical, Systemic framework),5 which is the basis for the work of the current commissions on spinal metastases.
Prognostic Tokuhashi (revised 2005) scale.
| Revised evaluation scale for the prognosis of spine metastases | |
|---|---|
| Characteristics | Score |
| 1. General status (functional status): | |
| Poor (EF 10%–40%) | 0 |
| Moderate (EF 50%–70%) | 1 |
| Good (EF 80%–100%) | 2 |
| 2. Number of extraspinal osseous mx: | |
| ≥3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| 3. Number of extraspinal osseous mx: | |
| ≥3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| 4. Metastases in major internal organs: | |
| Unresecable | 0 |
| Resecable | 1 |
| No metastasis | 2 |
| 5. Primary tumour: | |
| Lung, osteosarcoma, oesophagus, stomach, bladder, pancreas | 0 |
| Liver, unidentified | 1 |
| Others | 2 |
| Kidney, uterus | 3 |
| Rectum | 4 |
| Thyroid, breast, prostate, carcinoid tumour | 5 |
| 6. Paralysis: | |
| Complete (Frankel A, B) | 0 |
| Incomplete (Frankel C, D) | 1 |
| No paralysis (Frankel E) | 2 |
| Prognostic criteria according to score: | |
| 0–8 <6 months survival | |
| 9–11 ≥6 months survival | |
| 12–15 ≥1 year survival | |
Spinal instability neoplastic score.
| Spinal instability neoplastic score (SINS) | |
|---|---|
| Characteristics | Score |
| 1. Site: | |
| Transitional (Occ-C2, C7-T2, T11-L1, L5-S1) | 3 |
| Flexible spine (C3-C6, L2-L4) | 2 |
| Semi-rigid spine (T3-T10) | 1 |
| Rigid spine | 0 |
| 2. Pain: | |
| Yes | 3 |
| Occasional but not mechanical pain | 1 |
| Lesion with no pain | 0 |
| 3. Bone lesion: | |
| Lytic | 2 |
| Mixed | 1 |
| Blastic | 0 |
| 4. Al Radiographic spine alignment: | |
| Subluxation/movement present | 4 |
| de novo deformity (Cyphosis/escoliosis) | 2 |
| Normal alignment. | 0 |
| 5. Vertebral body collaps: | |
| >50% collaps | 3 |
| <50% collapse | 2 |
| No collapse with >50% of the body affected | 1 |
| None of the above | 0 |
| 6. Involvement of posterolateral elements: | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
| Prognostic criteria according to score: | |
| 0–6: stable | |
| 7–12: indeterminate or imminent instability | |
| 13–18: unstable | |
| SINS 7–18: ensure interconsultation to surgery | |
Today we consider a survival expectancy of less than 3 or 6 months as the threshold for offering invasive treatment, but how can we refuse treatment to patients when we cannot be sure of this life expectancy? Or how can we refuse treatment to patients with disabling pain or progressive neurological impairments because their life expectancy is less than 3 months?
This vision, established on knowledge and evidence that dates back to the first decade of this century, is also changing with the development of new oncological treatments based on genetic and molecular therapy (the most widely used scales, Tomita or Tokuhashi, do not include this consideration); changes in radiotherapy algorithms based on single major targeted doses (Stereotactic Body RadioTherapy [SBRT]; extracranial stereotactic radiosurgery) or with protons that achieve high percentages (70%–90%) of local control independently of the primary6 tumour or, in surgery, the change from the concept of “excisional” surgery to “separation” surgery associated with radiosurgery (SBRT), the former being mainly indicated in the treatment of radioresistant tumours. All of these are giving patients a better life expectancy, and oncologists and surgeons new therapeutic weapons.
In this sense of focusing surgical treatment on the patient's expectations and quality of life, it is worth evaluating the results of the study by Dea et al.7 in which they evaluated an international cohort of 466 patients, from countries of different cultures, with a survival prognosis of less than 3 months. Their results showed that 76.9% of patients were satisfied with their treatment at 6 weeks and none reported not recommending it. However, as expected, they found a greater number of adverse effects in the group of patients with lower life expectancy compared to those with higher life expectancy, effects which, when analysed, did not occur during intraoperative management (they were not associated with the “surgical burden” measured in duration of surgery or blood loss), but in the postoperative period.
For all these reasons, they conclude, and we agree, that the preoperative baseline clinical condition should be the main determinant of surgical indication rather than the expected survival. These indications should also take into account the concept of outcome effectiveness, with recommendation of the least aggressive possible.
Predictive scales reviewThis improvement in the survival of tumour patients with metastatic disease has been achieved thanks to a better understanding and treatment of the biology of metastasis (RANKL-RANK association mechanism and the development of drugs such as denosumab that block it by preventing the osteolysis process); the progress of new knowledge in immunogenetics which has made treatments with target drugs directed against tumour cells a reality, allowing better survival rates in classically more aggressive tumours such as lung, melanoma or ovarian tumours, and the improved knowledge of spinal biomechanics in the context of metastatic tumours in these patients. In recent years these factors have given rise to numerous studies reviewing predictive scales based on the analysis of success according to the area under the curve (AUC) technique. These studies show good predictive results (discrimination “c-statistic: patients who survive/death with the best result being “1” and a result equal to chance “.5”).
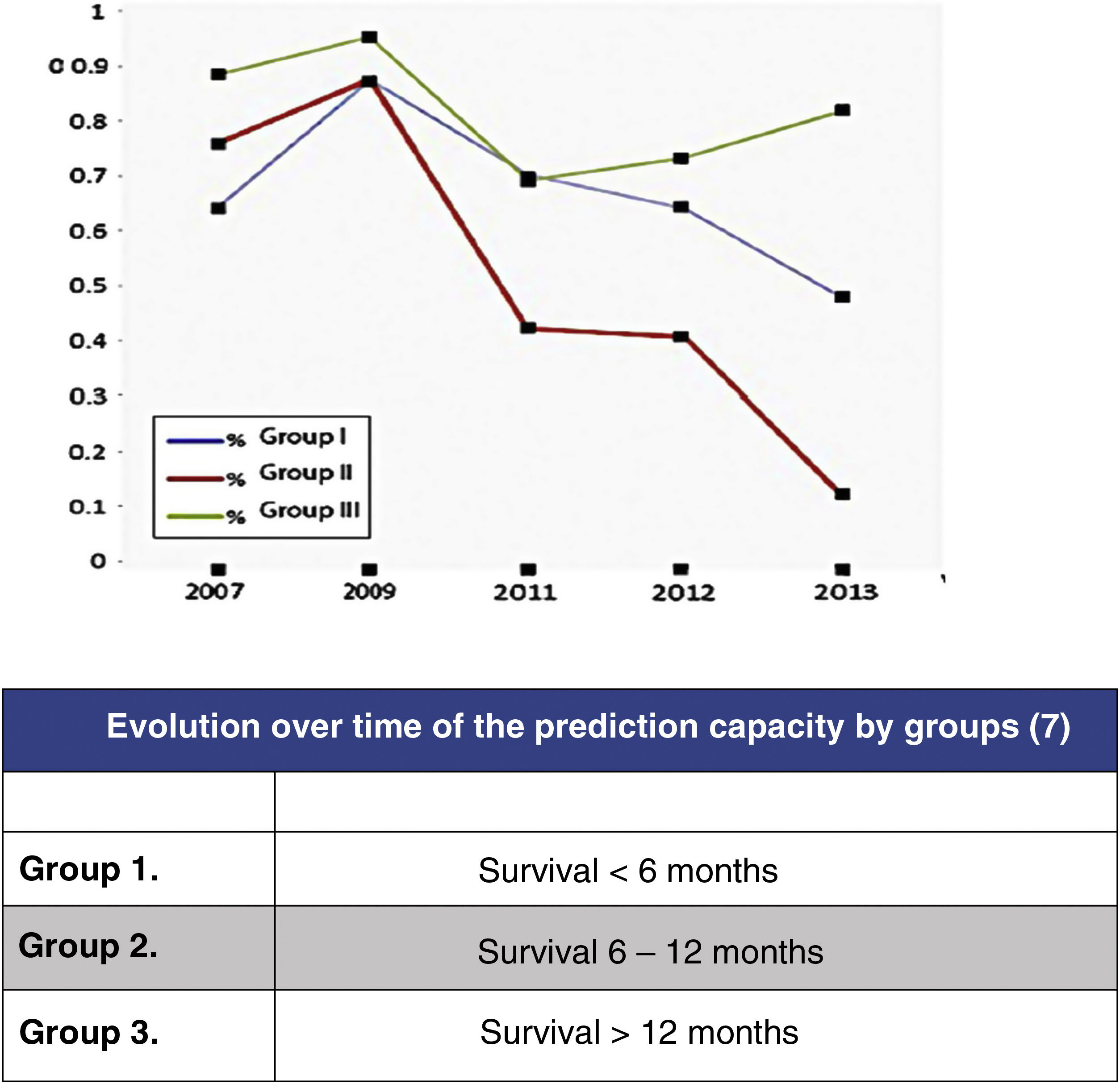
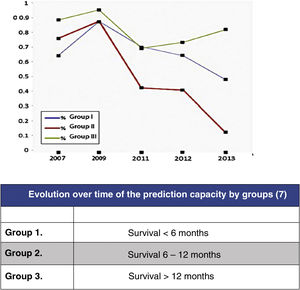
In this regard, the analysis of the predictive results of the Tokuhashi scale carried out by Zoccali et al.8 in which the reliability of the scale's survival prognosis from 2007 to 2013 is analysed (Fig. 2) may be highlighted. In this meta-analysis of the results of 10 studies involving 1, 686 patients, prediction is analysed according to the 3 groups defined by the Tokuhashi scale (group 1: survival <6 months, group 2: survival 6–12 months and group 3: survival >12 months), finding that if the overall predictive accuracy of the scale was 63%, it was very different depending on the group, being higher for the group with the longest survival (group 3: 77.21%), decreasing for group 1 (64.10%) and no higher than chance for group 2 (55.12%). The decrease in prediction has increased from the appearance of the scale in 2005 to the present day, reflecting the lack of sensitivity of the parameters in the groups with the poorest prognosis, which are those that have benefited most from the development of the most up-to-date treatments.
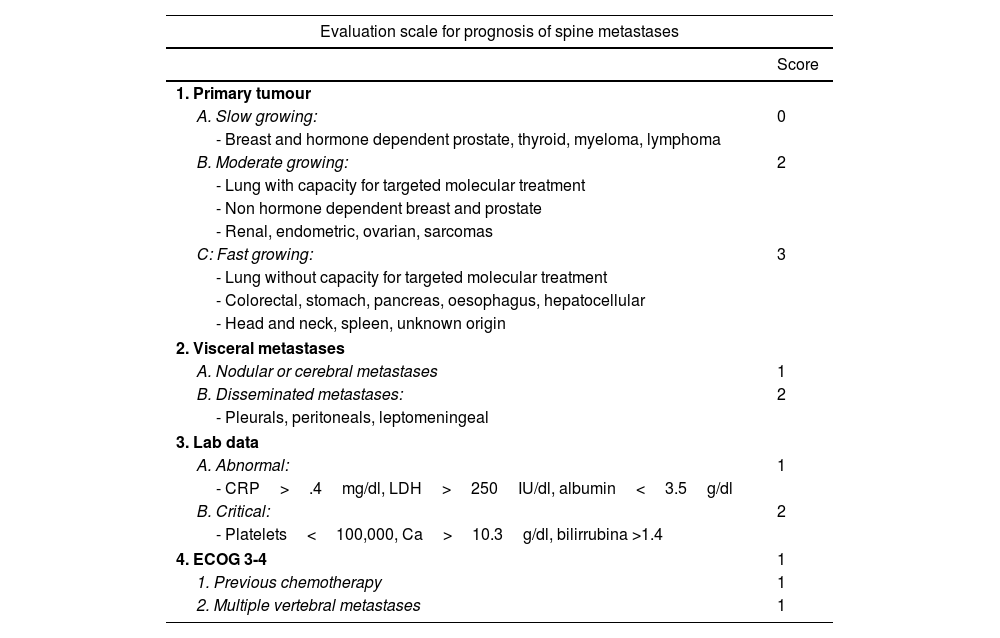
New scales and parameters are proposed to improve the reliability of predictions in higher risk groups, a first example of which is the one made by the group of Katagiri et al.9 (Table 3). For the first time this establishes prognostic differences depending on sensitivity to hormone-dependent treatment in the case of breast or prostate or with specific target drugs in lung cancer, and biochemical parameters are also included in the assessment. The scale, with a score between 0 and 10, in our view improves the prognostic results (>.75, <.25) for the high and low survival groups, as well as in the 6-month survival of patients classified as intermediate. Thus, the recommendations for patients with score 0–3, whose life expectancy is greater than one year in 91%, treatment with radiosurgery or combined with separation surgery would be indicated. However, for patients with a score between 7 and 10, with a 6-month survival expectancy of 27% and only a one-year expectancy of 6%, the most indicated option would be conventional radiotherapy combined with internal fixation in case of instability or neurological progression.
Katagiri8 prognostic scale.
| Evaluation scale for prognosis of spine metastases | |
|---|---|
| Score | |
| 1. Primary tumour | |
| A. Slow growing: | 0 |
| - Breast and hormone dependent prostate, thyroid, myeloma, lymphoma | |
| B. Moderate growing: | 2 |
| - Lung with capacity for targeted molecular treatment | |
| - Non hormone dependent breast and prostate | |
| - Renal, endometric, ovarian, sarcomas | |
| C: Fast growing: | 3 |
| - Lung without capacity for targeted molecular treatment | |
| - Colorectal, stomach, pancreas, oesophagus, hepatocellular | |
| - Head and neck, spleen, unknown origin | |
| 2. Visceral metastases | |
| A. Nodular or cerebral metastases | 1 |
| B. Disseminated metastases: | 2 |
| - Pleurals, peritoneals, leptomeningeal | |
| 3. Lab data | |
| A. Abnormal: | 1 |
| - CRP>.4mg/dl, LDH>250IU/dl, albumin<3.5g/dl | |
| B. Critical: | 2 |
| - Platelets<100,000, Ca>10.3g/dl, bilirrubina >1.4 | |
| 4. ECOG 3-4 | 1 |
| 1. Previous chemotherapy | 1 |
| 2. Multiple vertebral metastases | 1 |
| Prognostic criteria according to score (95%) | |||
|---|---|---|---|
| Score | 6 months | 12 months | 24 months |
| 0–3 | .981 | .914 | .778 |
| 4–6 | .740 | .493 | .276 |
| 7–10 | .269 | .060 | .021 |
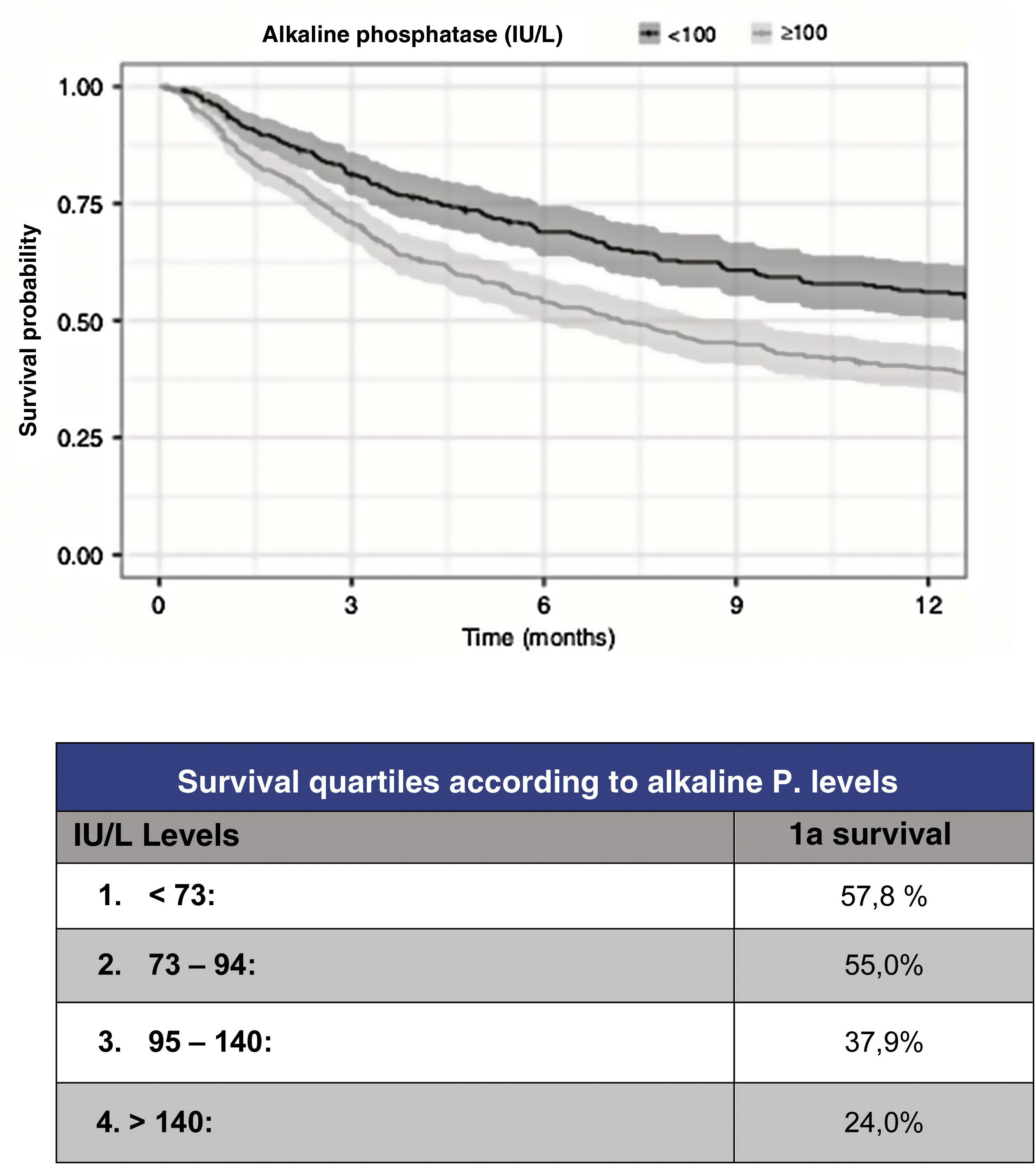
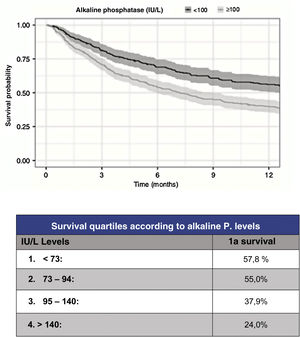
However, although the predictive capacity of these scales has improved to reach a statistic c=.78 for prediction at one year, their predictive capacity for the most severe patients has improved to the same extent. Thus, in order to improve the reliability of survival prediction, new, especially biochemical parameters have been proposed for inclusion. As a useful and simple example, Karhade et al.10 (Fig. 3) proposed serum alkaline phosphatase levels under 100 (c=.75) as an independent prognostic factor for survival longer than 6 months and/or metastatic tumour burden.
Prognosis according to alkaline phosphatase levels.9
FA is a metalloenzyme that is expressed on the surface of osteoblasts and whose level indicates increased activity. In osteolytic lesions it is produced secondarily in an attempt to compensate for destruction and in osteoblastic lesions by direct stimulation. FA is also a marker of intrahepatic bile duct obstruction due to liver metastases.
Regarding tumour metastatic burden, levels >100UI/l are associated with statistically significant differences (p<.001) for the presence of multiple spine metastases (102UI/l), presence of extra-spinal bone mx (105UI/l) or presence of visceral mx (105UI/l).
Finally, the prognostic role of FA in patients treated only with RT, CT or palliative measures has not been studied and neither have the changes in FA in patients treated with antiresorptive drugs before or after surgery.
New scales based on algorithmsOutstanding in this attempt to ensure survival prediction is the work of the Paulino et al.11,12 group from the Massachusetts General Hospital. It described a first algorithm, the “SORG normogram”, based on multiple parameters to reach a prediction of .70 at 30, 90 and 365 days.
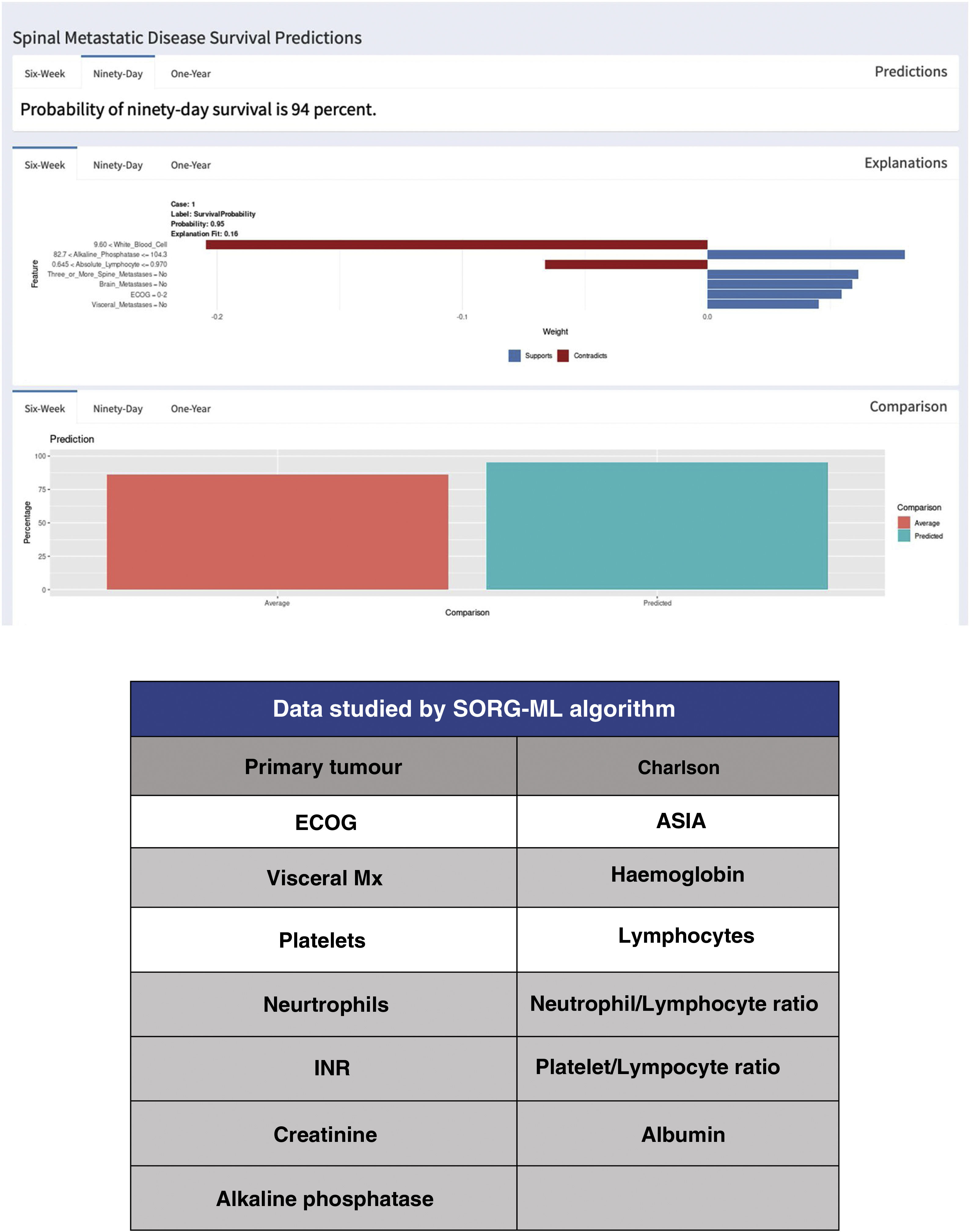
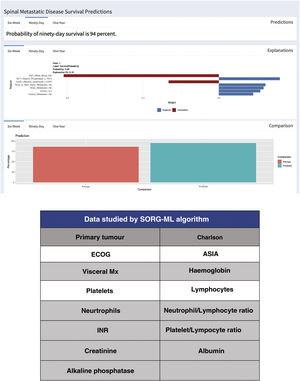
Since the publication of the “SORG normogram” staging, work has continued on more complete decision algorithms based on the capabilities of artificial intelligence (“machine learning”). Led by Karhade's group at Harvard Medical School, and joined by Sciubba's group at Johns Hopkins University School of Medicine,13,14 they created the survival prediction algorithm “SORG Machine-Learning” (SORG-ML), an algorithm based on 15 clinical and biochemical parameters which, entered into a computerised database, provide an automated prediction. This algorithm, externally validated,15 improves results in discrimination (c-statistic .75–.81 for 90 days and .77–.78 for one year), calibration, Brier score (mean squared difference between model predicted and observed) and decision curve.
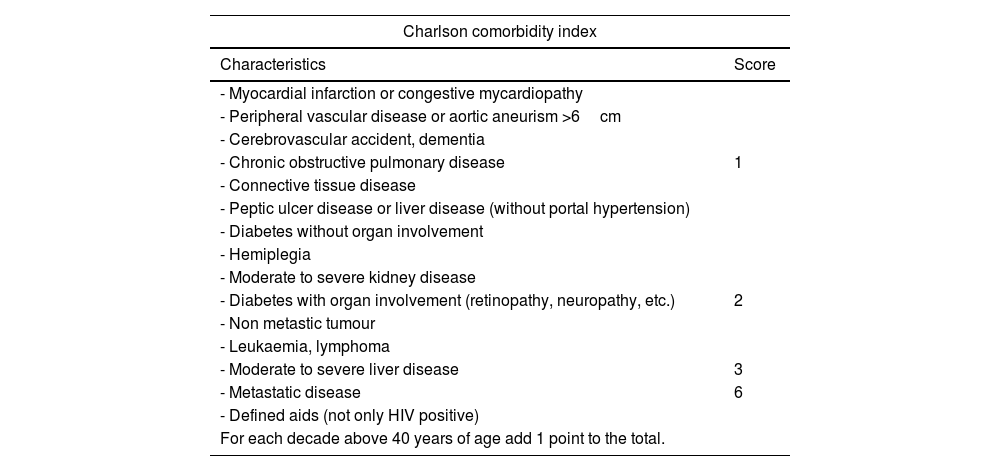
This study is based on the detection methodology described above, and on the study of a sample of 781 patients and 15 variables, both qualitative (primary tumour, ECOG or Charlson morbidity scale16,17) (Table 4) and quantitative (Alk. P levels, haemogram, etc.), creating a computerised algorithm with free access that assesses survival at 90 and 365 days on 15 parameters of different characteristics (https://sorg-apps.shinyapps.io/spinemetssurvival/) (Fig. 4).
Charlson comorbidity index.
| Charlson comorbidity index | |
|---|---|
| Characteristics | Score |
| - Myocardial infarction or congestive mycardiopathy | |
| - Peripheral vascular disease or aortic aneurism >6cm | |
| - Cerebrovascular accident, dementia | |
| - Chronic obstructive pulmonary disease | 1 |
| - Connective tissue disease | |
| - Peptic ulcer disease or liver disease (without portal hypertension) | |
| - Diabetes without organ involvement | |
| - Hemiplegia | |
| - Moderate to severe kidney disease | |
| - Diabetes with organ involvement (retinopathy, neuropathy, etc.) | 2 |
| - Non metastic tumour | |
| - Leukaemia, lymphoma | |
| - Moderate to severe liver disease | 3 |
| - Metastatic disease | 6 |
| - Defined aids (not only HIV positive) | |
| For each decade above 40 years of age add 1 point to the total. | |
HIV: human immunodeficiency virus.
Regarding mortality at 90 days, the prediction of the algorithm obtained a c=.83 with the most predictive variables being the primary tumour, preoperative albumin and ECOG grade. Regarding mortality at one year, it reached a statistic of c=.85.
External validation of the SORG-ML algorithm was made compared with 9 prognostic scales (Tokuhashi, revised Tokuhashi, Tomita, Bauer, revised Bauer, Katagiri, van der Linden, SORG classic and Sorg normogram) concluding that it presents a better prediction in mortality at 90 days (AUC=.75–.81) and similar for one year (AUC=.77–.78).
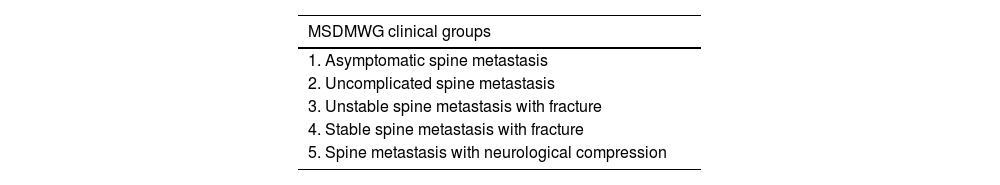
Our indications according to the NOMS conceptIn 2015, the Metastatic Spine Disease Multidisciplinary Working Group, a group that includes radiation oncologists, interventional radiologists and surgeons, proposed consensus treatment guidelines based on 5 clinical groups,18 corrected in 201919 (Table 5), in accordance with this multidisciplinary concept in the approach to the treatment of metastatic patients. Following this idea and the updated literature, in which the patient's general condition and stability are the first factors to be evaluated when it comes to the indication, rather than the symptomatology itself, we have proposed the following treatment groups:
Clinical groups of the Metastatic Spine Disease Multidisciplinary Working Group.
| MSDMWG clinical groups |
|---|
| 1. Asymptomatic spine metastasis |
| 2. Uncomplicated spine metastasis |
| 3. Unstable spine metastasis with fracture |
| 4. Stable spine metastasis with fracture |
| 5. Spine metastasis with neurological compression |
MSDMWG: Metastatic Spine Disease Multidisciplinary Working Group.
- a.
Palliative radiotherapy: only indicated in patients with a poor systemic status, ECOG 3–4 and/or neurological involvement ASIA A of over 48h evolution.
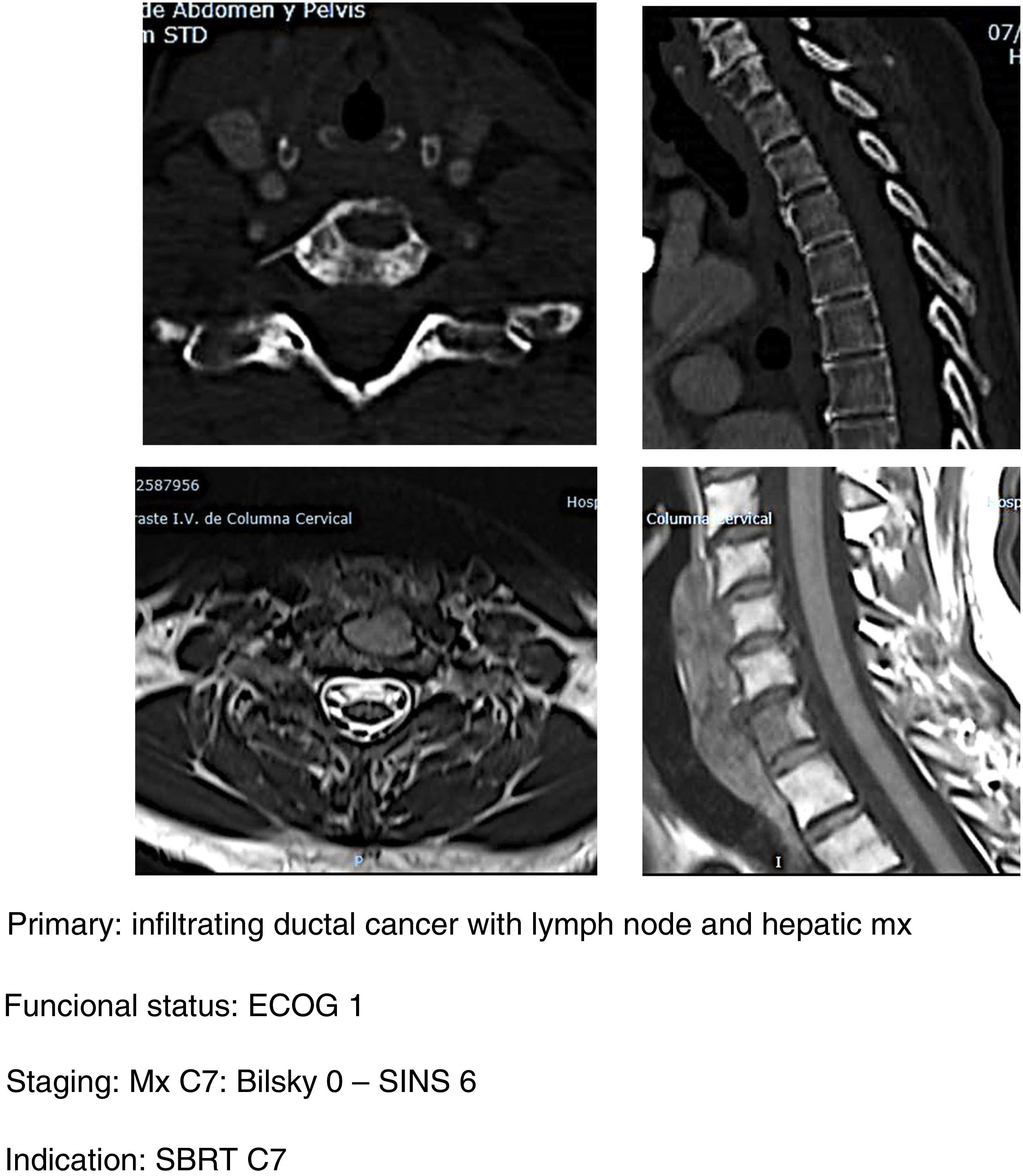
- b.
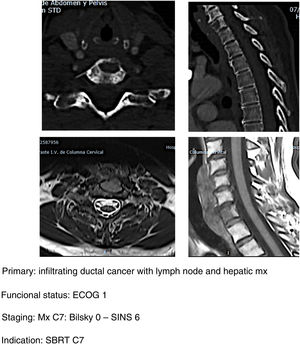
SBRT (Fig. 5a–d): our indication for single high-dose stereotactic radiosurgery is established in patients with good clinical status ECOG 1–2, stable with SINS <6 and with epidural compression <2. Preferably life expectancy >6 months and with single metastasis.20 It is important to take into account the determination of spinal stability prior to the indication of SBRT as a single treatment even in patients without epidural compression. In a study of patients21 with vertebral mx without epidural compression, prior RT or surgery undergoing SBRT they found that patients with SINS≥11 were associated with a higher number of adverse effects in the radiated area (vertebral fractures, admission for pain, emergency or scheduled surgery, new neurological symptoms and/or epidural compression). A higher frequency of adverse effects is also observed when comparing SBRT versus conventional multifraction Minimally aggressive techniques (Fig. 6, a–h): the combination of RT with percutaneous techniques such as ablation or cementation is a useful option for fragile patients. Our indication is the same in patients up to ECOG 2, with slow growing tumours and multiple metastases or metastases which progress despite satisfactory control of the systemic disease, particularly in areas of thoracolumbar hinge zones with SINS up to 9 (according to the considerations indicated by Lam et al.21) and preferably with low degrees of spinal cord compression (Bilsky 1C).
Some considerations in the application of this technique must be taken into account in order to avoid the complications described with its application (up to 20%). Contraindications for the use of cementation are the involvement of the posterior wall or the presence of a neurological lesion. Regarding ablation, a minimum of 5mm must be established between the ablation area and the spinal cord in order to avoid lesion and to assess the cementation in order to avoid the development of a fracture.
Preventive treatment: SINS 7–12In the evaluation of the mechanical stability of patients assessed by the SINS scale, the guidelines for action are clear for the “stable” (0–6) and “unstable” (13–18) groups. However, for the “potentially unstable” category (7–12), which accounts for 75% of the patients consulted,19 there is no consensus agreement on the parameters for deciding whether or not to stabilise.
In this sense, Pennigtong et al.22 carried out a review of the therapeutic decisions indicated in a series of patients classified in this group, and of their clinical characteristics. They present a series of patients with a median SINS of 9 (IQR: 7–10 and mode 10) with a Tokuhashi of 8 (IQR: 7–11) and 75% of unstable lesions.
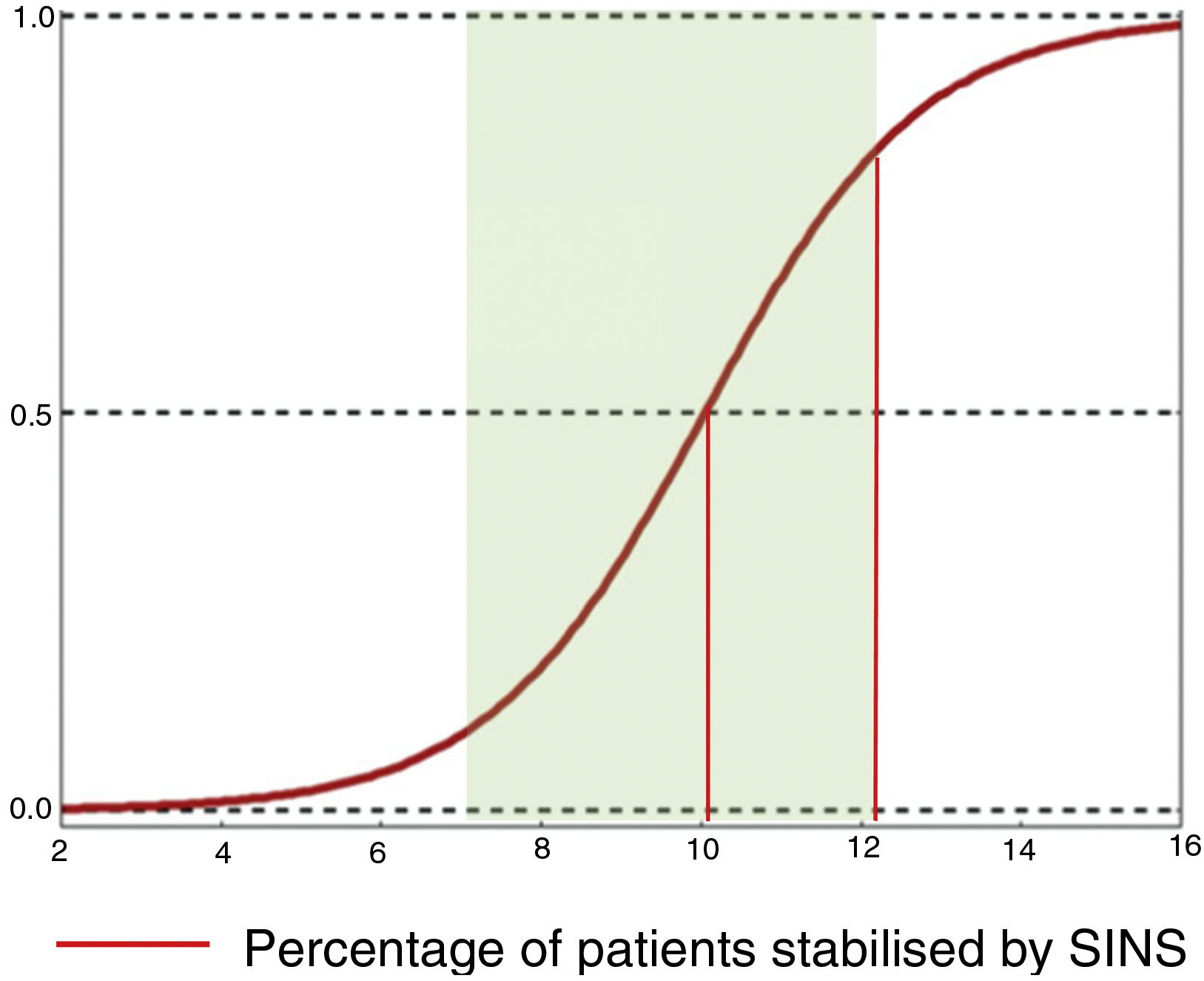
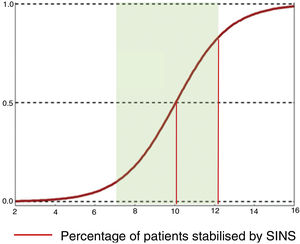
Their conclusions were that 79% of patients with SINS 10-12 were operated on, compared with only 11% with SINS 7–9. The reasons for operating on patients with SINS 7–9 was an epidural compression ESCC 2–3, while patients with high SINS with low survival probability (ECOG 3 and multiple lesions) were not operated on. The operated patients, irrespective of their SINS, were significantly younger, had a higher Tokuhashi, thus associated with longer survival and also a higher Karnofsky. A sub-analysis in these patients also found a higher lytic lesion involvement and pain. This finding is similar to that given by Versteeg et al.,23 who also give cut-offs of 10.7 vs. 7.2 (Fig. 7).
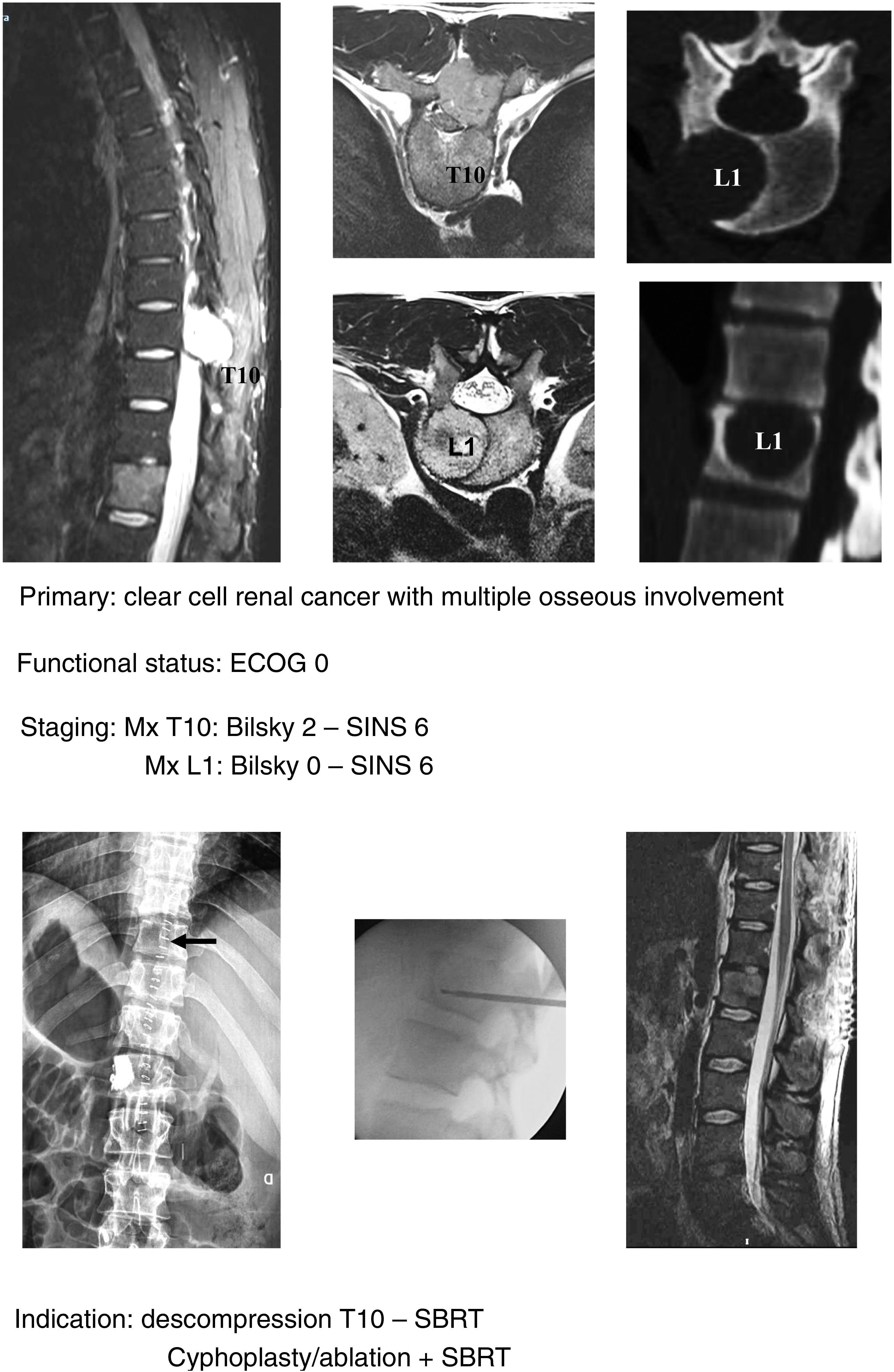
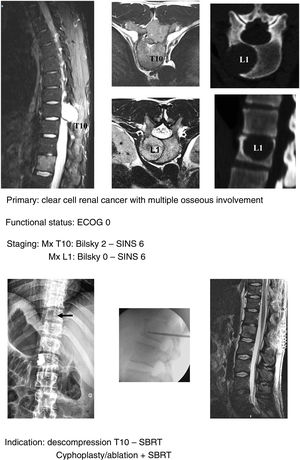
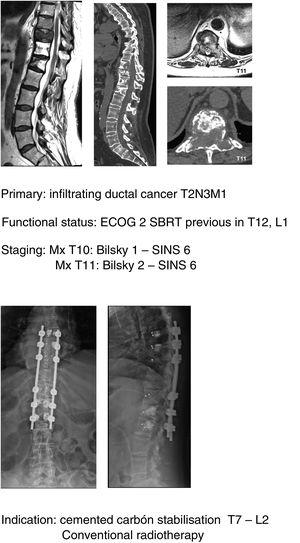
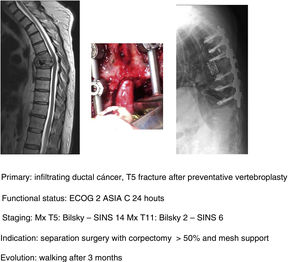
In keeping with this, our unit is particularly interventionist in patients with SINS 9–12, especially in patients with multiple sites or who have undergone previous radiotherapy. The main limiting factor for us is the general status of the patient and secondly the neurological condition. Estimated survival is much less important with our main aim being to ensure quality of life and neurological status during survival (Fig. 8a–f).
Separation surgeryFor many groups, including ours, during the last decade of the last century and the first decade of the present century, the indicated surgical treatment for patients with single metastasis was en bloc vertebrectomy described by Roy-Camille et al. and popularised by Tomita et al.24,25 whose aim was to perform surgery “as compartmental as possible”, transferring the excisional concept of oncological surgery of the extremities to the spine. The objections to this treatment lie, firstly, in the characteristics of the metastatic disease itself and the presence of “skip metastases” metastases not detectable at the time of indication and the extension of the tumour into the epidural space (layer D in the classification of Weinstein et al.,26) which implies that this compartmental resection is impossible except in patients with Tomita's type 1 intraosseous involvement.27
This concept changed radically in the second decade of this century with the appearance of stereotactic radiosurgery (SRS or SBRT) which allows the total application of the dose in single fractions, minimising the exposure of the tissues at risk, especially the spinal cord. This achieves a direct cytotoxic effect and local control of up to 98% at 2 years, changing the objectives of radiotherapy from traditional pain relief to ablative radiotherapeutic treatment of vertebral metastasis28 and, therefore, from surgical indications based on the need for vertebral tumour reduction or excision (corporectomies or vertebrectomies).
However, these high doses with a tumour-killing effect need a safety margin, estimated to be about 2mm, to be delivered, which is why the concept of “hybrid therapy” is defined, in which surgery aims to create this margin to allow safe treatment with radiosurgery in a second phase.
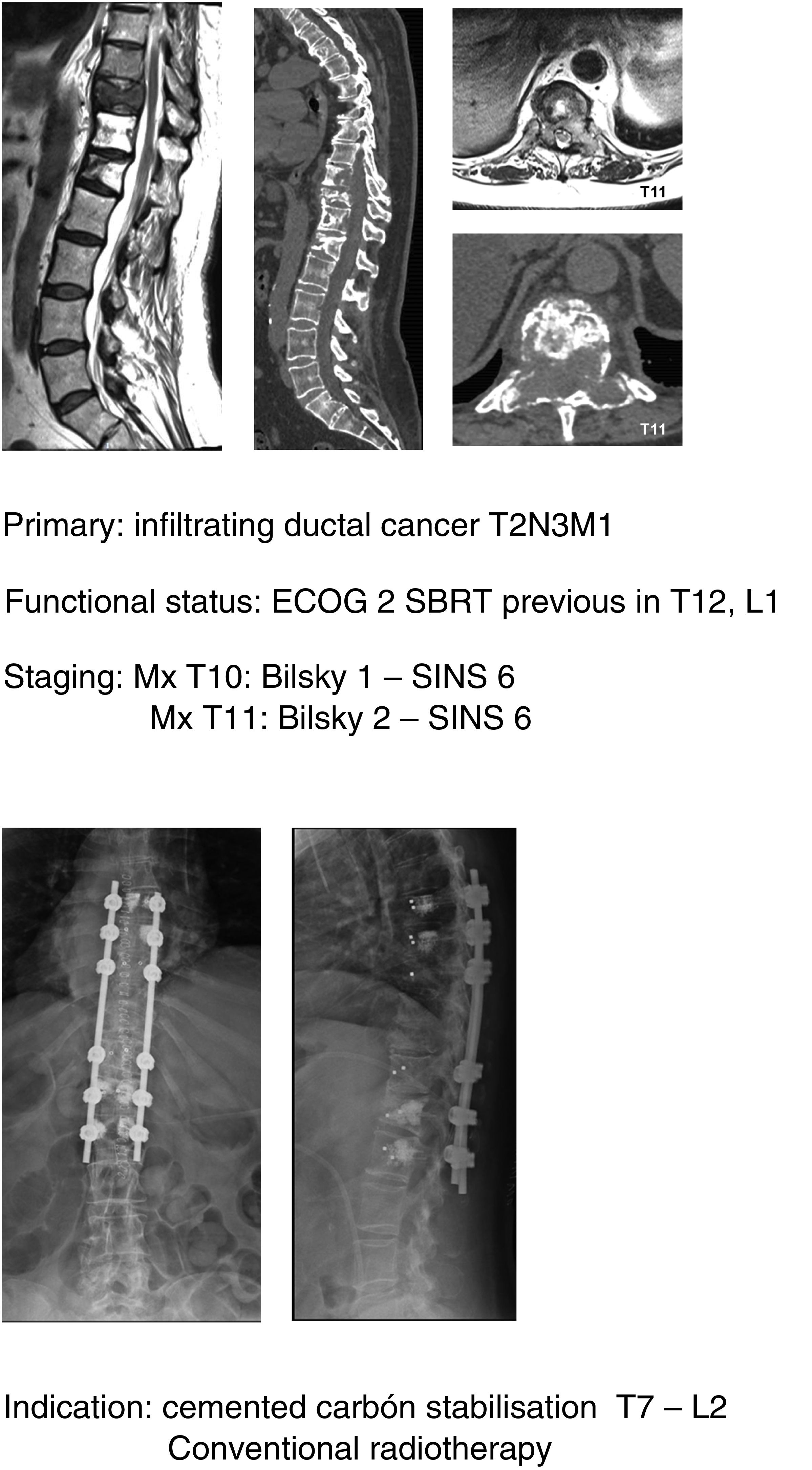
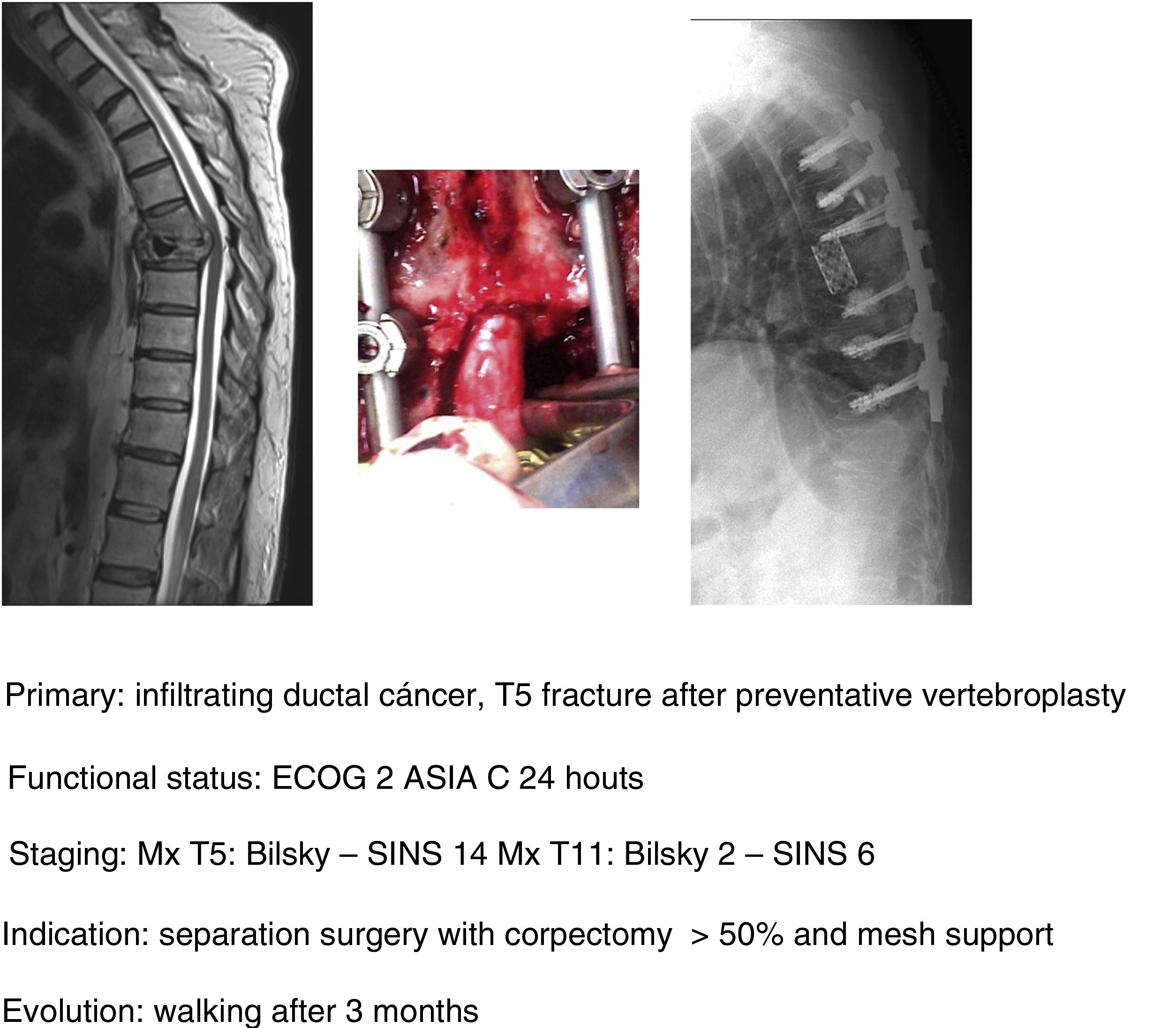
In their article Barzilai et al.29 give a detailed description of the technique, which is based on a first step of instrumented stabilisation, at least 2 levels above and below the lesion in order to restore a mechanical instability present or secondary to the removal of bone from the posterior elements. A second step of decompression that must take into account the use of technical gestures that avoid putting pressure on the compression, using drills as much as possible (areas free of tumour involvement) and being careful in the intracanal manipulation of the Kerrison heel. This posterior part should end with the achievement of an access corridor to the anterior component that allows the resection of 20% of the vertebral body, the separation of the thecal sac from the posterior common vertebral ligament and the depression of the tumour component in order to obtain a circumferential separation of the dura. Excision of the remaining vertebral tumour within the body or in paraspinal tissues is not necessary as they fall within the treatment field of radiosurgery. Anterior support is only needed in case of a vertebral body resection of more than 50% (Fig. 9a–c). In our unit, protocolised preoperative tumour embolisation is almost routinely performed, especially in patients with hypervascular tumours.
In recent years, various technological innovations have improved the separation technique. From the surgical point of view, the development of implants made of carbon fibres reinforced with PEEK® (Fig. 10a–g) has led to an improvement in the quality of the planning and application of radiotherapy by avoiding the scattering effect induced by titanium implants on ionising radiation and particles without reducing the safety offered by the implants in mechanical terms,30 as well as improving the quality of postoperative use of MRI.31
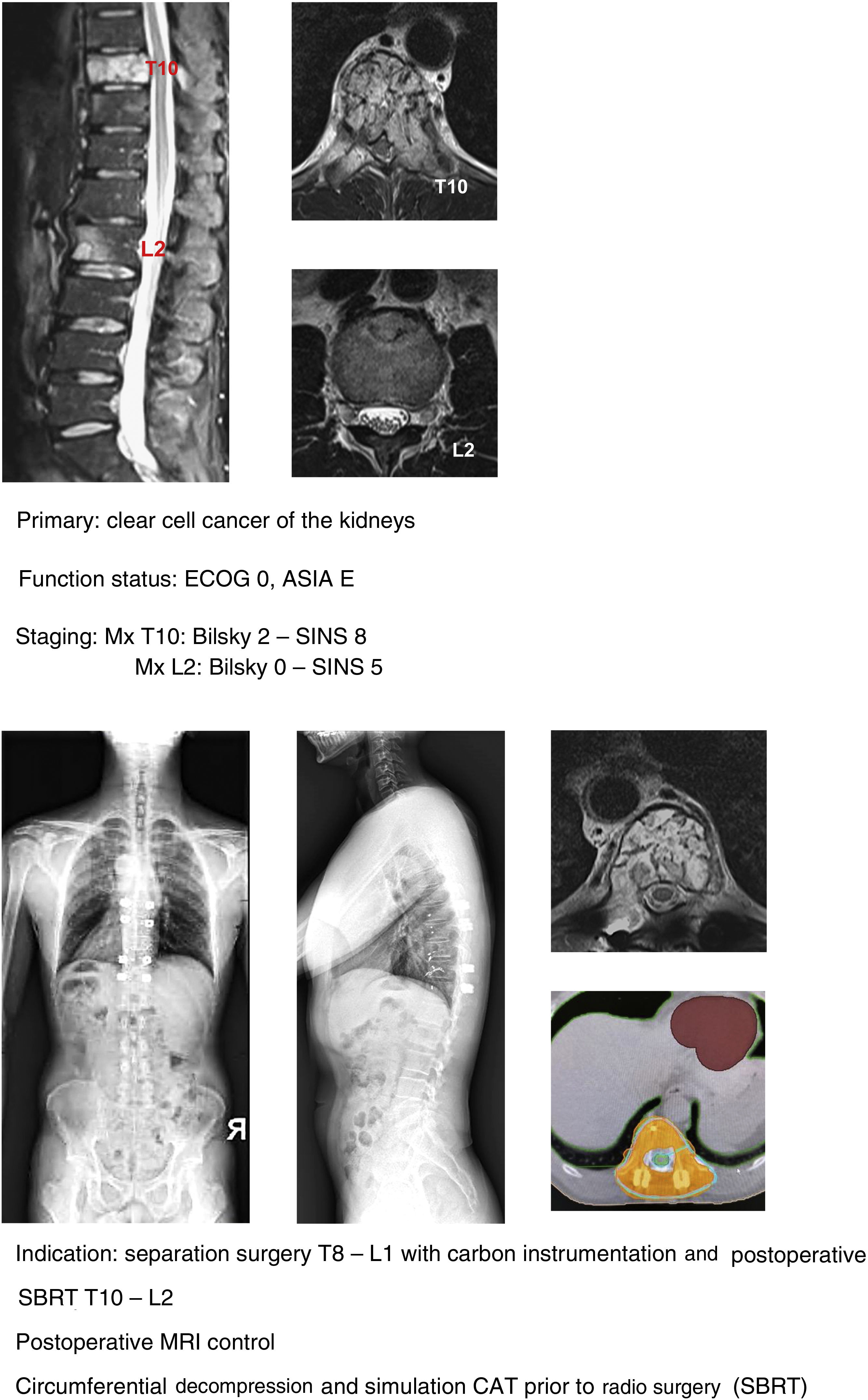
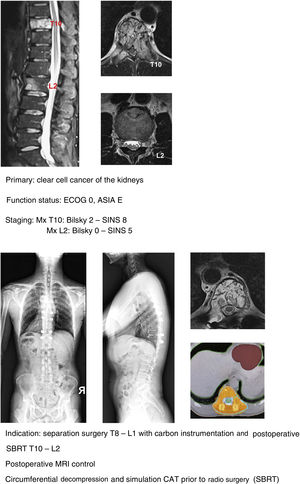
In our unit the technique of choice in the treatment of single metastasis is SBRT radiosurgery when spinal cord compression is Bilsky <2 and the hybrid protocol described for separation surgery with carbon instrumentation combined with early SBRT (2 weeks) in Bilsky ≥2 compression cases. According to the literature, the indication for the hybrid technique does not depend on the type of tumour or age of the patient, but above all on the patient's functional status, the degree of spinal cord injury and the prognosis according to the NOMS.
Spinal cord compression associated with neurological impairmentIn this section on “preventive” treatment, we also wanted to include the indications, especially in terms of time, of spinal cord compression with neurological impairment. In this regard, we should highlight the work of Patchel et al.1,32 and Cole et al.32 in which they report a 62% recovery of ambulatory capacity after decompression surgery plus stabilisation and radiotherapy performed before 48h in patients with incomplete or progressive lesions, or that of Fan et al.,33 the latter performed on a group of 43 patients with complete paraplegia (34 Frankel A and 9 Frankel B) in which they observed that those Frankel B patients operated on in a period of less than 48h also improved by at least 2 grades on the scale. None of the Frankel A patients regained ambulation postoperatively. This recommendation is set at 24h, the treatment recommendations given by the NICE guideline.34
Regarding other predictors, Laufer35 established as a poor predictor of functional recovery the established vesicle dysfunction and, in contrast, presented a muscle balance of 3. No relationship has been found between functional recovery with other types of variables such as age, sex and type (aggressiveness) of the tumour. No statistically significant relationship was found with an increase in complications, infections or hospital stay either, compared with preoperative neurological involvement.36
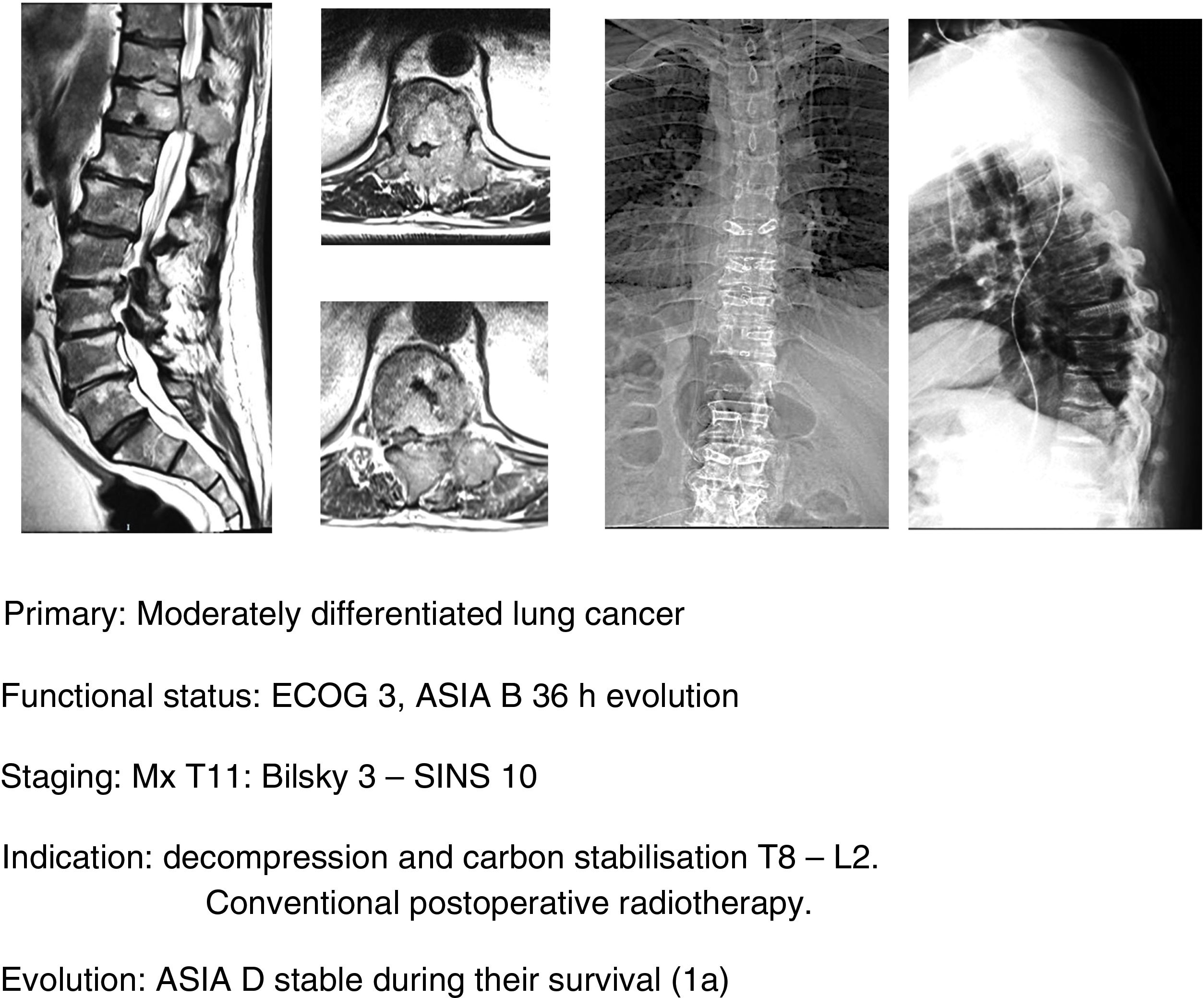
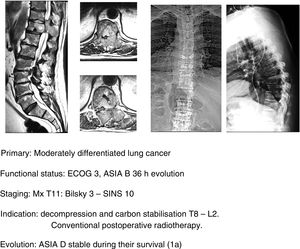
In this sense, our approach is the same as studies that have been commented upon,1,32–34 indicating, firstly, that general medical treatment (analgesia, thromboprophylaxis) be used, specifically with large doses of dexametasone in 16mg/24h doses, neurological status control and surgery in ECOG 0–2 patients who present with a neurological status up to ASIA B of under 48h evolution, regardless of the type of tumour and above all if this is associated with the impossibility of radiosurgery (Bilsky≥2) and/or the presence of oligometastasis. Early decompression may prevent the cascade of vascular changes which trigger ischaemia and spinal infarction,37 and thereby the ability of reversibility of the neurological involvement, facilitating a particularly significant qualitative neurological improvement (Fig. 11a–e).
Level of evidenceLevel of evidence I.
FundingNo funding was received for this article.
Conflict of interestsThe authors have no conflict of interests to declare.