The spine is the third most frequent location for metastatic disease, after the lung and liver. On the other hand, the most frequent bone tumours are metastases and the spine is the main location. A review of the different imaging techniques available, both radiological and nuclear medicine, and the morphological appearance of spinal metastases in each of them is performed. Magnetic resonance imaging is the best imaging modality for detection of spinal metastases. It is important to make the differential diagnosis between vertebral fracture of osteoporotic and pathological cause. Spinal cord compression is a serious complication of metastatic disease and its assessment by imaging through objective scales is decisive for estimating spinal stability and therefore establishing treatment. Lastly, percutaneous intervention techniques are briefly discussed.
La columna vertebral es la tercera ubicación más frecuente para la enfermedad metastásica, después del pulmón y el hígado. Por otra parte, los tumores óseos más recurrentes son las metástasis, siendo la columna su principal lugar de localización. En este trabajo se realiza una revisión de las diferentes técnicas de imagen disponibles, tanto radiológicas como de medicina nuclear, y de la apariencia morfológica de las metástasis de columna en cada una de ellas. La resonancia magnética es la mejor modalidad de imagen para la detección de metástasis en la columna. Es importante efectuar el diagnóstico diferencial entre fractura vertebral de causa osteoporótica y patológica. La compresión medular es una complicación grave de la enfermedad metastásica y su valoración mediante imagen a través de escalas objetivas es determinante para la estimación de la estabilidad de la columna y, por consiguiente, para establecer el tratamiento. Por último, se comentan brevemente las técnicas de intervencionismo percutáneo.
Cancer is the second leading cause of death in the world. The spine is the third most frequent site for metastatic disease, after lung and liver. Primary tumours are mainly breast, lung, prostate, thyroid, and kidney,1 but in 13% of cases, the primary tumour is unknown.2 However, the most recurrent bone tumours are metastases, and the spine is their main location. Most spinal metastases occur in middle-aged and geriatric patients.3
Spinal tumours are classically grouped into three categories according to their anatomical distribution: extradural, intradural-extramedullary, and intramedullary. Most metastases are found in the extradural compartment, which comprises the bony spine and epidural region. They are frequently located in the vertebral bodies, usually extending to the pedicles. The most affected segment is the thoracic spine (70%) and with less recurrence the lumbar segments (20%), and the cervical and sacral segments (10%).4
Metastases initially infiltrate the vertebral bodies, due to their bone marrow composition and high vascularisation, and from there spread to the posterior arch. The most common route of dissemination is haematogenous; direct, lymphatic, and subarachnoid extensions are less frequent. The venous route, especially Batson's venous plexus, appears to be more common than the arterial route. Arterial metastases are usually located in the vicinity of the vertebral plates, whereas tumours metastasising via the epidural Batson's venous plexus affect the posterior third of the vertebral body. The cancellous bone is invaded first and, as infiltration progresses, the cortical bone is invaded, the latter favouring pathological fracture and vertebral instability.3
Spinal metastases can cause axial pain and functional limitations as a result of pathological fractures. They are also often accompanied by soft tissue masses causing radicular and spinal epidural involvement, resulting in radiculopathy and myelopathy. Ten percent of patients with vertebral metastases will develop spinal cord compression.5
MethodsThis paper reviews the different imaging techniques available, radiological, and nuclear medicine, and the morphological characteristics of vertebral metastases on each.
ResultsThe initial literature search on imaging of vertebral metastases yielded 1044 articles, of which 192 were reviews. Most of the papers were discarded because they focused on atypical or rare findings, because they emphasised central nervous system abnormalities in the spine, or because their publication date was not recent, as imaging techniques evolve rapidly.
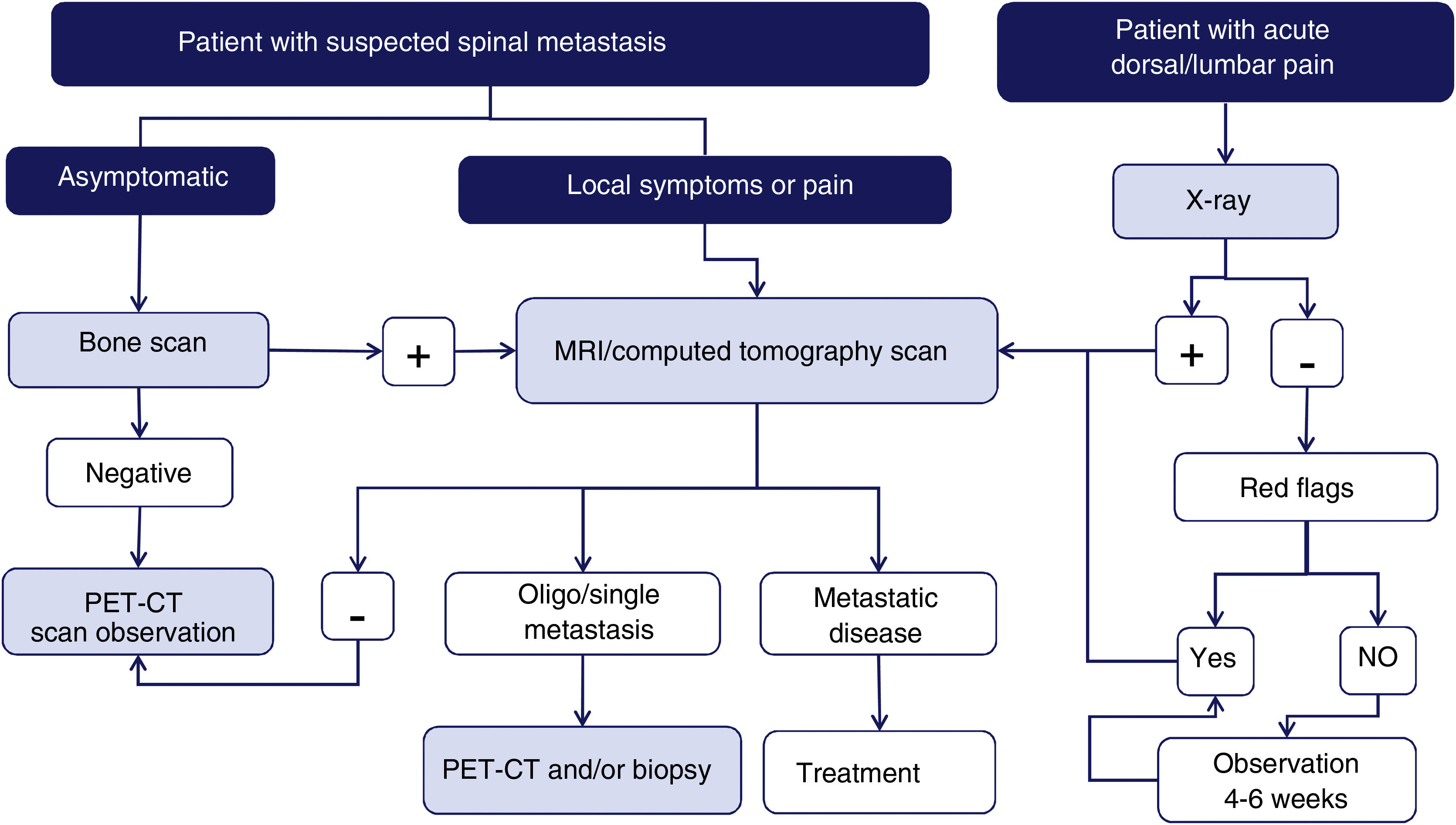
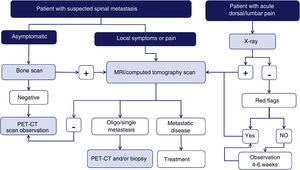
Imaging techniques: diagnostic algorithmAccording to a meta-analysis on the diagnosis of spinal metastases, magnetic resonance imaging (MRI) is the best imaging method for the detection of spinal metastases, performed per patient and per lesion. The meta-analysis compared the sensitivity, specificity, and diagnostic accuracy of MRI with the other tests: positron emission tomography (PET), single photon emission tomography (SPECT), computed tomography (CT), and bone scintigraphy (BS)6 (Fig. 1).
Diagram of diagnostic algorithm for spinal metastases. Warning signs or red flags include: 1. Suspicion of tumour involvement; 2. Suspicion of infection; 3. Suspicion of fracture; 4. Pain with inflammatory rhythm; 5. Cauda equina syndrome, progressive or severe neurological deficit; 6. Low back pain with very severe pain and progressing intensity; and 7. Subacute or chronic low back pain with radicular irradiation with treatment failure.
Plain X-ray is the primary imaging technique in the diagnostic approach to spinal pathology because it is technically easy to perform, low cost, and widely used. Its main indication is to rule out pathological fracture in patients with known symptoms and an acute clinical picture.4 However, it is not very sensitive for the early detection of metastases, with a high percentage of false negatives. A mass larger than 1cm in diameter and a loss of trabecular bone of at least 50% is required for the detection of a lytic lesion and therefore it is not suitable as a screening technique.1
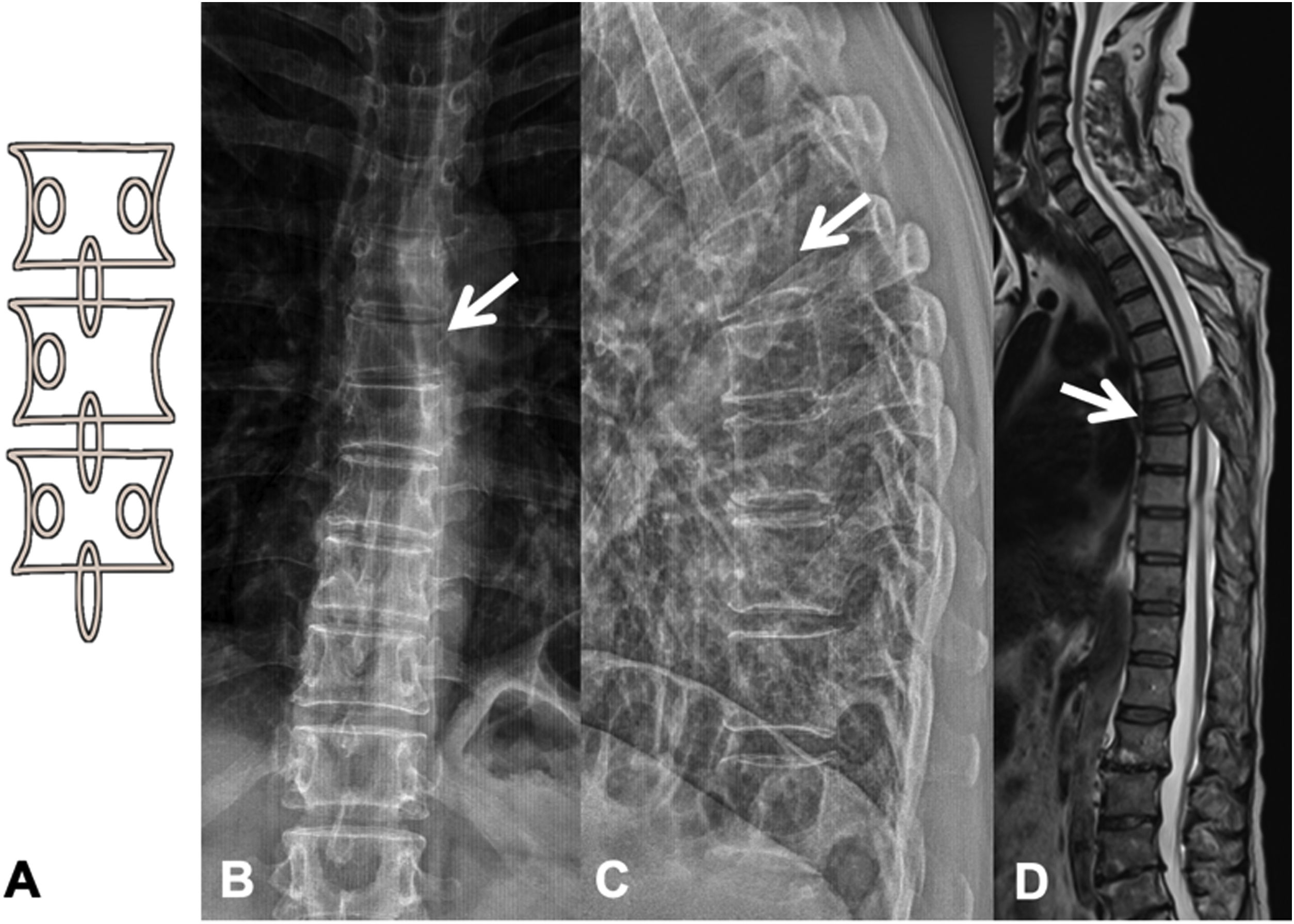
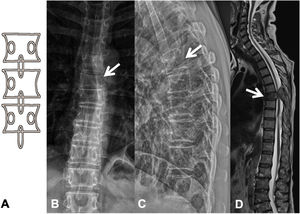
Metastases can be osteolytic (breast, lung, colon, thyroid, and kidney), blastic (prostate and neuroblastoma), or mixed (breast and lung). The most frequent are pure lytic lesions, visualised as loss of bone density, which present with a geographic, moth-eaten, or permeative pattern. Cortical involvement is identified as a blurring of the contour of the vertebral body, it is a very specific sign of tumour involvement and allows the risk of pathological fracture to be assessed. The risk is high if the tumour destroys 50% of the cortex. Common causes of pathological fracture, in order of decreasing frequency, are breast, lung, and prostate metastases. The winking owl sign on the anteroposterior projection of the spine indicates a lytic lesion involving the vertebral pedicle, characteristic of metastasis. The vertebral body represents the owl's head, the absent pedicle simulates the winking eye, the normal contralateral pedicle simulates the open eye, and the spinous process resembles the beak7 (Fig. 2).
Pathological dorsal fracture due to lung carcinoma metastasis. (A) Diagram of the winking owl sign; (B) X-ray in AP projection of the dorsal spine with asymmetrical vertebral wedging T7, more significant on the left side, with image in the vertebrae due to lysis of the left pedicle (arrow); (C) X-ray in lateral projection of the dorsal spine with vertebral wedging T7 (arrow); (D) X-ray in lateral projection of the dorsal spine with vertebral wedging T7 (arrow); (C) X-ray of the dorsal spine with asymmetrical vertebral wedging T7 (arrow): MRI of the entire spine with sagittal T2-weighted sequence, showing pathological fracture of T7 with bulging of the posterior wall, involvement of the posterior arch with epidural mass causing spinal cord compression (arrow). AP: anteroposterior; MRI: magnetic resonance imaging; X-ray: plain X-ray.
Blastic metastases are detected as homogeneous or heterogeneous sclerosed areas and are most frequent in prostate and gastric tumours, myeloma, and lymphoma.3 The ivory vertebra sign refers to an increase in opacity of a vertebral body, which maintains its size and contours, with adjacent intervertebral discs preserved. Differential diagnoses include metastases, Paget's disease, and lymphoma.8
Computerised tomographyX-ray is the initial imaging test, but due to the complex anatomy of the vertebrae, multidetector CT (MDCT) is more useful for assessing the location and characteristics of bone involvement.9 Furthermore, CT can recognise a bony metastatic lesion up to six months earlier than X-ray. Multiplanar 2-D and 3-D reconstructions with great anatomical detail are possible by axial volumetric acquisition of the images with millimetre-thick slices. Although CT provides excellent image quality and high spatial resolution, metastatic lesions without significant bone destruction may be missed, because it is less sensitive and specific than MRI. Furthermore, cortical destruction is particularly difficult to assess when severe osteoporotic or degenerative changes are present.6
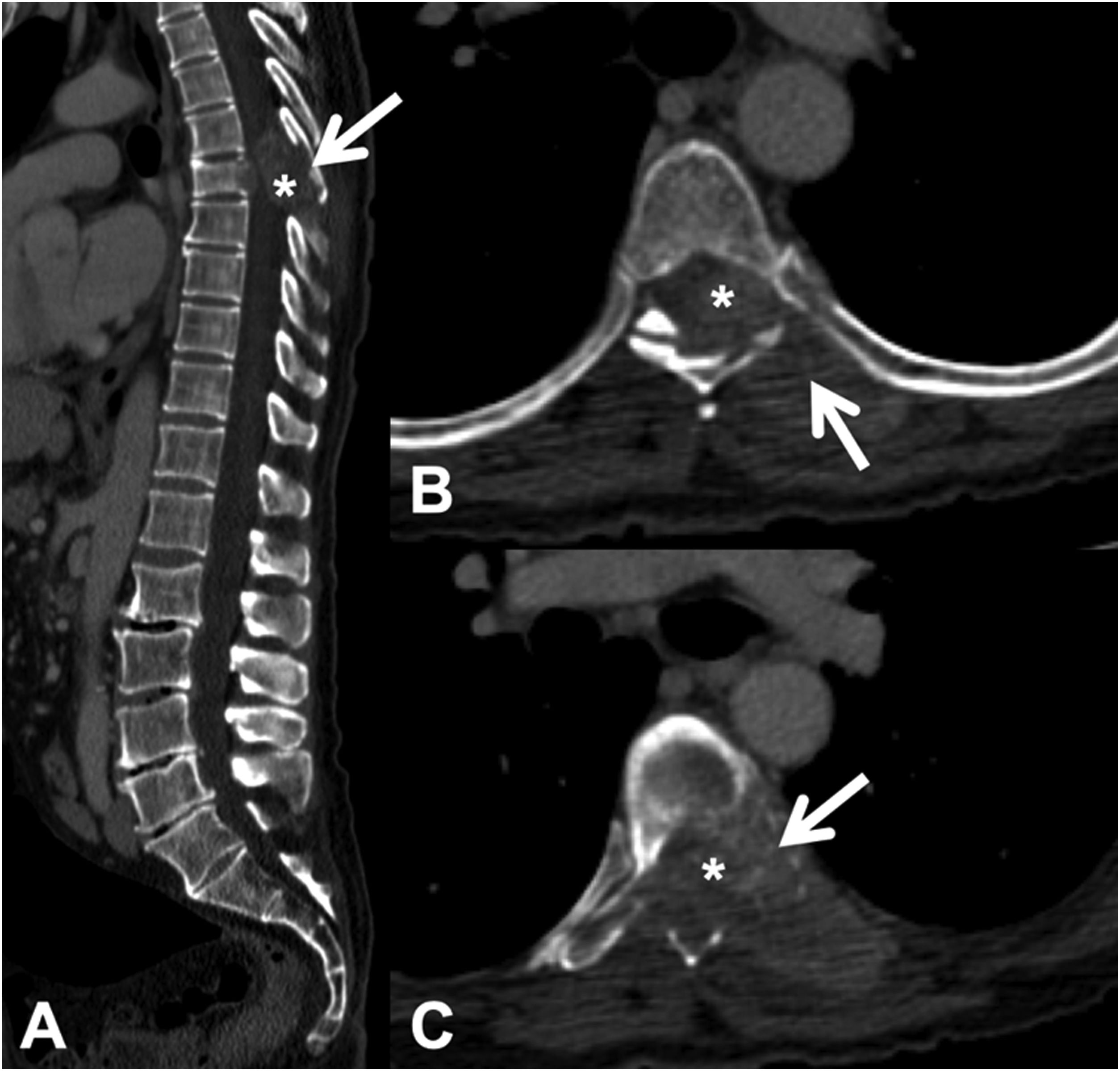
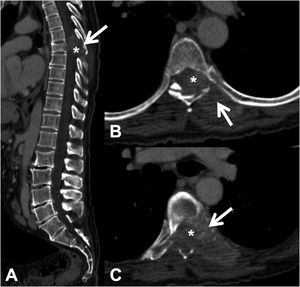
CT detects infiltration of both the cortical and the bony trabeculae of lytic lesions. It also allows detection of an associated epidural soft tissue component, which presents as an epidural mass displacing the thecal sac or occupying the conjunctival foramen1 (Fig. 3).
Pathological dorsal fracture due to lung carcinoma metastasis. Dorsolumbar CT with intravenous contrast. (A) 2D reconstruction in the sagittal plane, showing pathological T7 fracture with bulging of the posterior wall, involvement of the posterior arch (arrow) and epidural mass (asterisk); (B and C) axial slices at the level of the lesion, showing lytic lesion in the vertebral body, left pedicle and posterior arch (arrow), with epidural and paravertebral soft tissue component contrast uptake, and invading the spinal canal (asterisk). CT: computed tomography.
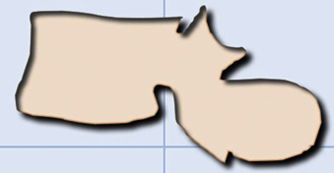
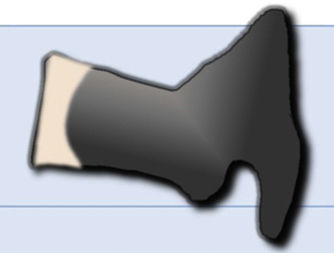
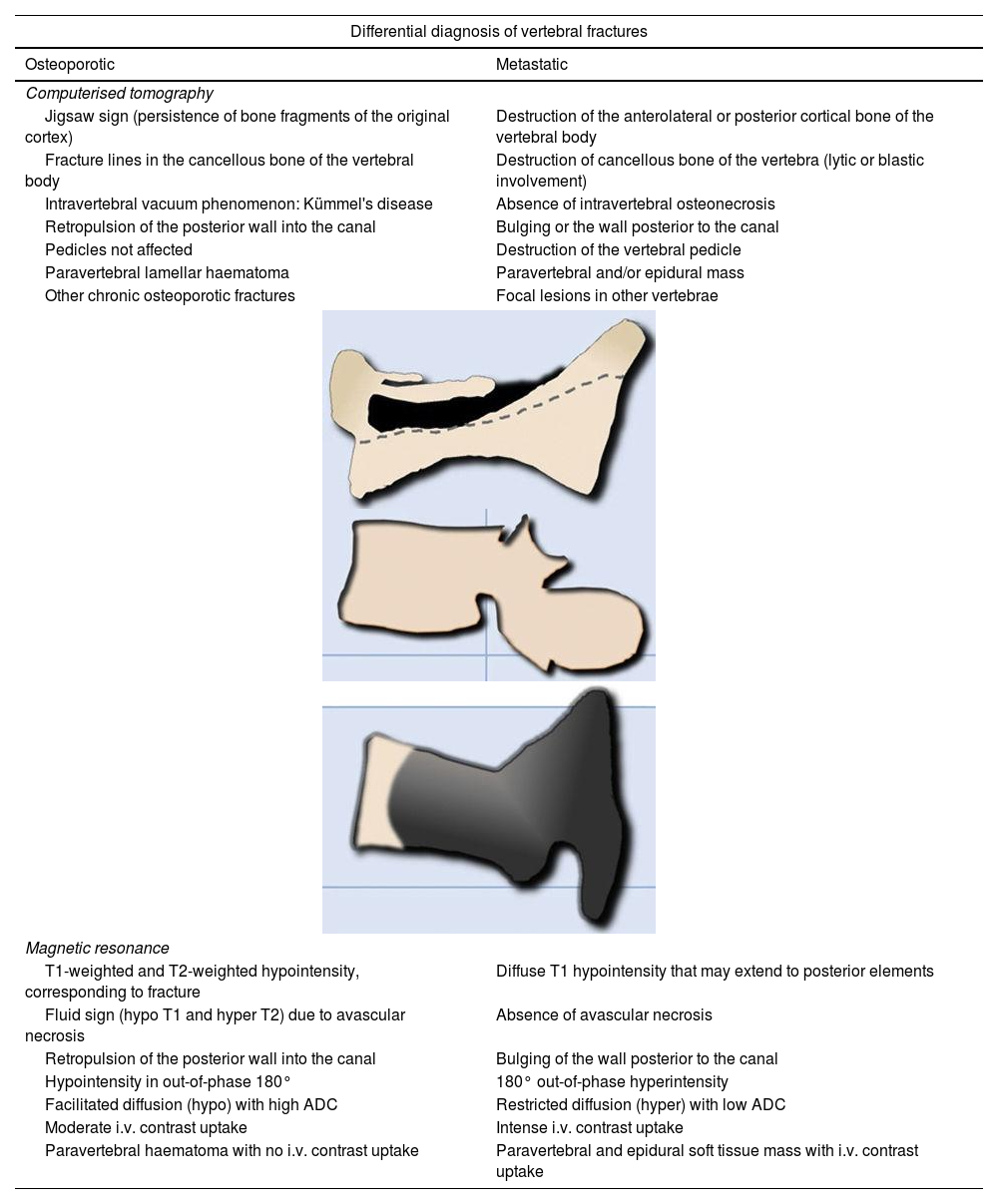
It is difficult to distinguish between acute vertebral fractures of osteoporotic and metastatic cause, both of which cause pain and may compress the spinal cord. The morphological findings associated with these two types of fractures, which allow a differential diagnosis between the two, are summarised in Table 1.3,10
Differential diagnosis of vertebral fractures by CT and MRI.
| Differential diagnosis of vertebral fractures | |
|---|---|
| Osteoporotic | Metastatic |
| Computerised tomography | |
| Jigsaw sign (persistence of bone fragments of the original cortex) | Destruction of the anterolateral or posterior cortical bone of the vertebral body |
| Fracture lines in the cancellous bone of the vertebral body | Destruction of cancellous bone of the vertebra (lytic or blastic involvement) |
| Intravertebral vacuum phenomenon: Kümmel's disease | Absence of intravertebral osteonecrosis |
| Retropulsion of the posterior wall into the canal | Bulging or the wall posterior to the canal |
| Pedicles not affected | Destruction of the vertebral pedicle |
| Paravertebral lamellar haematoma | Paravertebral and/or epidural mass |
| Other chronic osteoporotic fractures | Focal lesions in other vertebrae |
| Magnetic resonance | |
| T1-weighted and T2-weighted hypointensity, corresponding to fracture | Diffuse T1 hypointensity that may extend to posterior elements |
| Fluid sign (hypo T1 and hyper T2) due to avascular necrosis | Absence of avascular necrosis |
| Retropulsion of the posterior wall into the canal | Bulging of the wall posterior to the canal |
| Hypointensity in out-of-phase 180° | 180° out-of-phase hyperintensity |
| Facilitated diffusion (hypo) with high ADC | Restricted diffusion (hyper) with low ADC |
| Moderate i.v. contrast uptake | Intense i.v. contrast uptake |
| Paravertebral haematoma with no i.v. contrast uptake | Paravertebral and epidural soft tissue mass with i.v. contrast uptake |
ADC: apparent diffusion coefficient; CT: computerised tomography; i.v.: intravenous; MRI: magnetic resonance imaging.
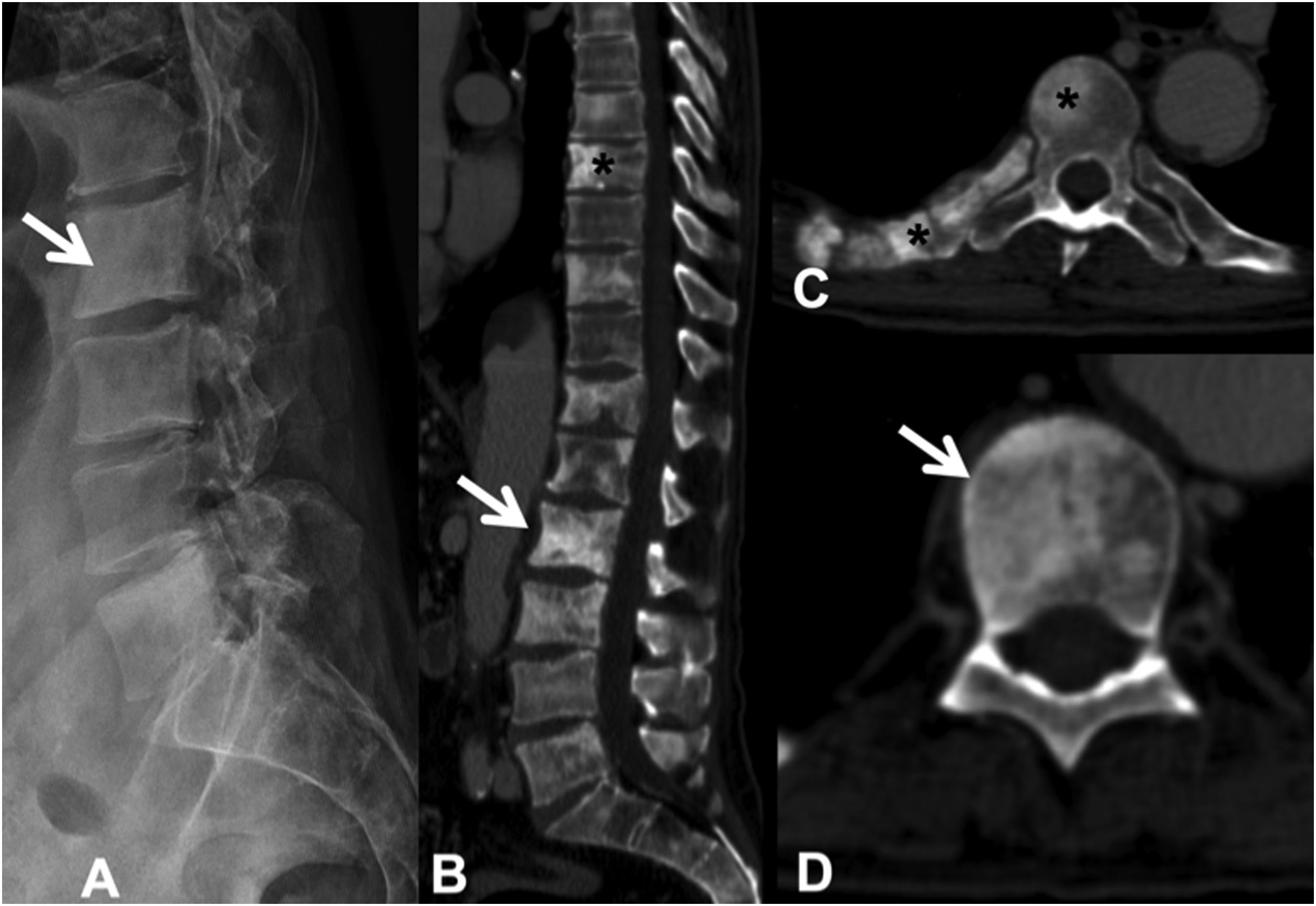
Sclerotic lesions are less frequent, are of high attenuation (although less than cortical bone) and heterogeneous, prevent visualisation of the trabeculae, and may be associated with periosteal reaction; it is rare for them to have cortical involvement or soft tissue mass. It is possible to differentiate untreated blastic metastases from enostoses or bone islands by attenuation measurements. Metastases show an attenuation of less than 885 Hounsfield units (HU), while enostoses exhibit a higher attenuation, reaching 1060HU, which enables a reliable diagnosis.11 Osteoblastic flare is the appearance of a sclerotic rim around an initially lytic lesion or sclerotic lesions not previously detected on X-ray or CT, during the follow-up of an oncological patient, with other signs of partial response to treatment. It does not indicate tumour progression, but rather the healing of pre-existing lesions12 (Fig. 4).
Multiple dorsal and lumbar sclerosing metastases due to prostate carcinoma. (A) Lateral X-ray of the lumbar spine, showing some of the existing sclerotic metastases (arrow); (B) CT scan of the dorsolumbar spine with dorsal (asterisk) and lumbar (arrow) sclerotic metastases; (C and D) sclerotic metastases at various levels, in the posterior arch and right rib (asterisk), and in the vertebral body (arrow). CT: computerised tomography; X-ray: plain radiography.
Full body CT is useful in patients with suspected vertebral metastases to detect the primary tumour. CT is also useful in planning surgical intervention, as well as in the choice of type and extent of instrumentation.4 It is useful in directing guided biopsy13 or percutaneous ablation and is used in treatment planning for stereotactic body radiotherapy (SBRT).
Advances in dual-energy CT (DECT) are promising,14 from the data set obtained, virtual post-processing of mono-energetic extrapolation can be performed, which substantially reduces the beam hardening artefact produced by the metal and improves visualisation of the instrumentation and adjacent structures. Algorithms are used as another method to reduce metal artefacts; they segment the metal data, removing damaged data, which are reconstructed to generate a corrected image. The used of dual energy with calcium-free virtual technique to detect oedema and pathological bone marrow infiltration also increases the diagnostic yield.15
CT myelography is a useful technique in patients who cannot undergo MRI (pacemakers, claustrophobia, etc.) or due to the presence of metallic instrumentation causing significant paramagnetic artefact. It allows assessment of bone integrity as well as the contents of the thecal sac and has the additional benefit of allowing cerebrospinal fluid (CSF) analysis. It can characterise spinal cord compression and the degree of myelographic block.1
Magnetic resonanceMRI is considered the most useful imaging modality for assessing metastatic disease of the spine, and is more sensitive than X-ray, CT, and nuclear medicine scans. Its sensitivity is due to its excellent contrast resolution between tissues; it provides information on the number, size, and location of metastases, invasion of the spinal canal, degree of spinal cord or radicular compression, involvement of adjacent soft tissues and large vessels.4
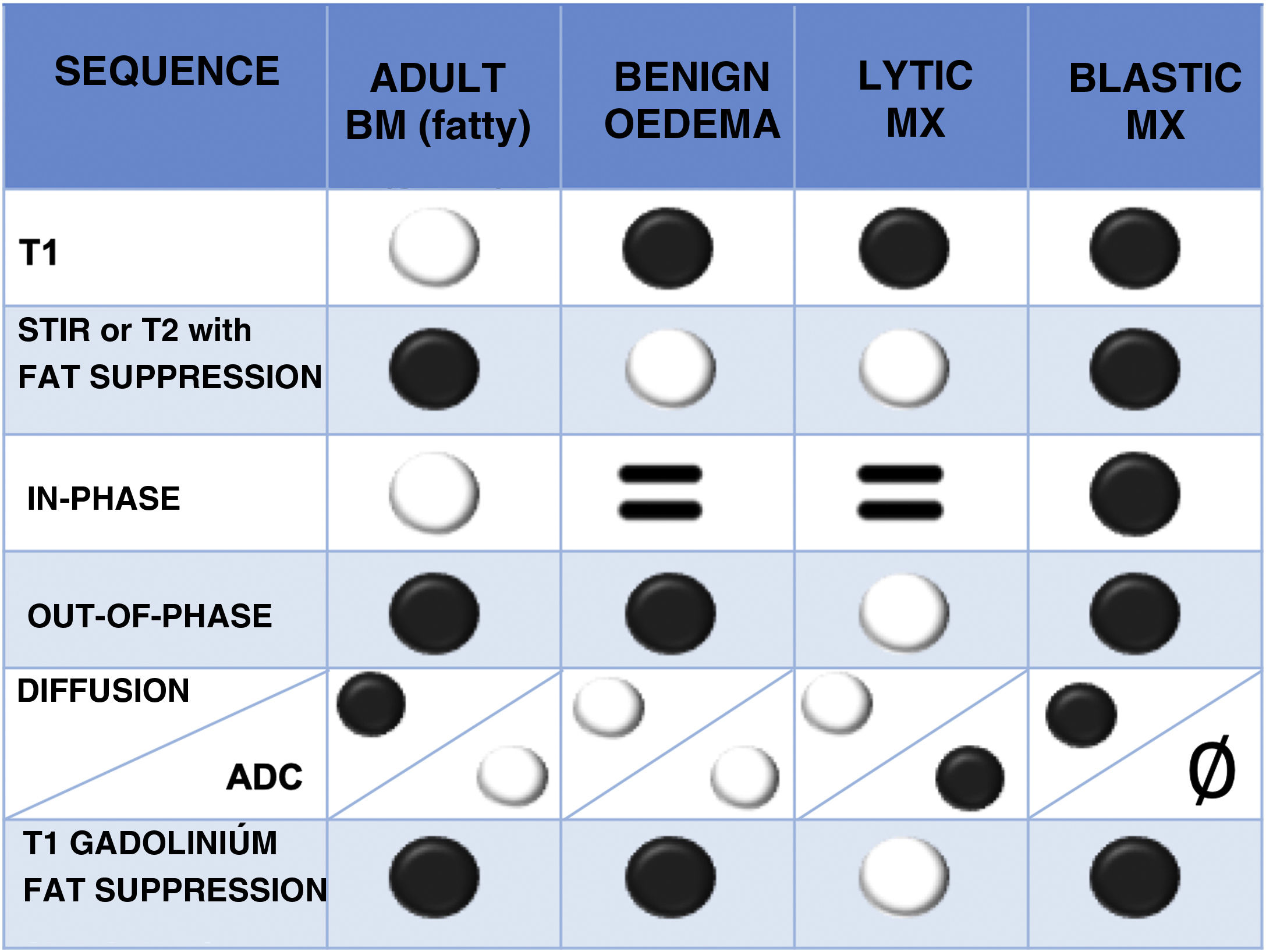
Normal bone marrow contains both fat and water (yellow marrow is 80% fat and 15% water, and red marrow 40% fat and 40% water). In infiltrative disorders the fat disappears, therefore sequences showing differences between fat and water signal are useful.1 The most important sequences are briefly described below (Table 2 and Fig. 5):
- -
T1 spin echo (SE) sequence: fatty bone marrow contains a high percentage of the latter and shows a marked hypersignal on T1, any focal lesion infiltrating fat reveals hyposignal and is therefore easy to detect. This explains why the T1 sequence is very useful and is often the first to be used. Haematopoietic marrow contains water as well as fat, is hypointense relative to fat, but hyperintense relative to normal muscle. Metastases, on the other hand, show a characteristic T1 hyposignal that is more marked with respect to muscle and intervertebral discs.16 Diffuse infiltration of the vertebral bone marrow produces such a homogeneous signal hypointensity that it may initially create the impression of a normal study, for correct assessment, it must be compared with the signal from discs and muscles.1
- -
T2-weighted sequences: metastases show variable T2 signal, depending on whether they are lytic (hyperintense, due to their high water content) or blastic (hypointense). Metastases often have a T2 bright signal around them (halo sign).17
- -
Fat suppression techniques: there are many, the most classical is the Short Tau Inversion Recovery (STIR) sequence that has the advantage of being a robust technique compared to more modern and specific fat saturation sequences such as Spectral Presaturation with Inversion Recovery (SPIR) and Spectral Attenuated Inversion Recovery (SPAIR), which are very susceptible to paramagnetic artefacts due to metallic instrumentation material. A modern and increasingly used sequence is the DIXON technique, which allows fat-specific saturation and can obtain images where water and fat molecules are in-phase and out-of-phase. This information is mathematically combined to obtain four sequences: in-phase (water+fat); out-of-phase (water−fat); fat-only (in-phase−out-of-phase), and water-only (in-phase+out-of-phase). It is becoming more widely used because in the same sequence a T2 sequence without and with fat saturation is available, saving time in the acquisition of the study. It also allows a qualitative and quantitative study of bone marrow composition. There is evidence that low fat fractions of the lesion under study are associated with malignancy, given that oncological processes, when replacing bone marrow, significantly reduce its fat content. In out-of-phase images, a signal loss of less than 20% with respect to the in-phase images is considered diagnostic of a malignant lesion. Conversely, a signal loss of more than 20% on phase-contrast images compared to in-phase images is diagnostic of a benign lesion.18–20
- -
Diffusion-weighted (DW) imaging: This sequence assesses the diffusion of water from tissues. Tissues with higher cellularity have more intra- and intercellular membranes (i.e., diffusion barriers) and therefore more diffusion restriction (hypersignal) and reduced apparent diffusion coefficient (ADC) values (hyposignal). The usefulness of diffusion for differentiating benign from metastatic lesions in the spine is debated, but quantitative assessment of ADC in vertebral bodies is generally considered an objective and comparable parameter for differentiating malignant from benign vertebral tissue.21,22 In addition, the diffusion sequence can demonstrate response to treatment. The latter reduces the cellularity of the lesion and therefore there is less restriction of diffusion (less hypersignal than pre-treatment) and increased ADC values.23
- -
Intravenous contrast sequences: these are T1-weighted sequences, producing hypersignal in vascularised tissues, such as lytic metastases. The enhancement pattern is variable in sclerosing metastases. Fat saturation techniques are required to increase the visibility of enhanced lesions, as normal bone marrow is hyperintense on T1-weighted images, as are enhancing lesions. Contrast administration is also very useful to assess the epidural soft tissue component, the tumour can spread to the anterior epidural space without affecting the meningovertebral ligament, resulting in the “draped curtain sign”.1
- -
Whole-body MRI: T1-weighted and STIR-weighted sequences are used, obtaining whole-body images, which are fused in the craniocaudal axis. This is a new alternative for detecting metastatic disease, multiple myeloma, and bone lymphoma.24
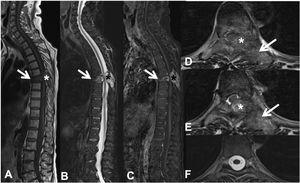
Pathological dorsal T7 fracture due to lung carcinoma metastasis. Dorsolumbar MRI with intravenous contrast. (A) T1 sequence in the sagittal plane, where there is a pathological T7 fracture with bulging of the posterior wall and hypointense signal, involvement of the posterior arch (arrow) and epidural mass (asterisk); (B) sagittal T2 sequence with hyperintense metastasis; (C) T1 sequence with intravenous gadolinium and fat suppression, the metastasis has contrast uptake; (D and E) axial T2 sequence with involvement of the posterior vertebral arch (arrow) and epidural soft tissue component producing spinal cord compression (asterisk); (F) axial T2 sequence in normal section, without compression of the spinal parenchyma. MRI: magnetic resonance.
The differential imaging diagnosis of vertebral body metastases would include benign haemangioma, discogenic changes in the vertebral discs, and discitis/osteomyelitis. Vertebral haemangiomas are usually well-demarcated benign vascular tumours, hyperintense on T1-weighted images and of variable signal on STIR, depending on the proportion of fatty and vascular elements, and associated contrast uptake, with thick vertical trabeculae resembling honeycombing on X-ray and CT. Discogenic changes of the vertebral disc plates usually occur on both sides of the disc. Acute intravertebral disc herniation or Schmorl's node shows oedema signal (hyposignal on T1 and hypersignal on T2/STIR). In discitis/osteomyelitis, there is oedema and erosion of the vertebral discs with intradiscal fluid and patchy enhancement. Bone metastases do not normally cross the disc space from one vertebral body to the next.1
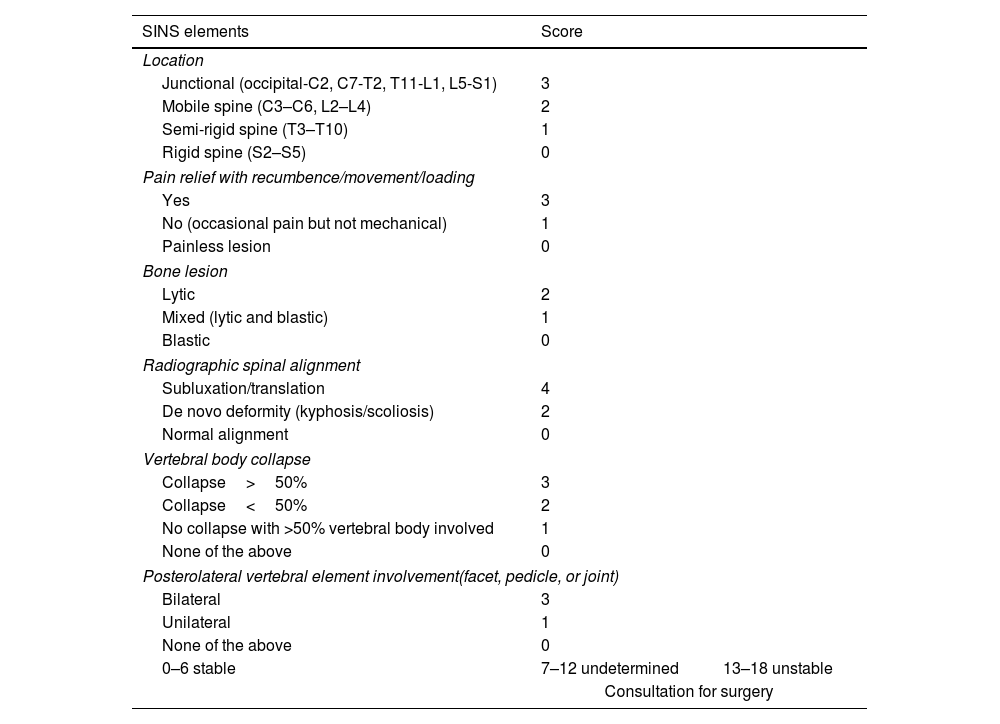
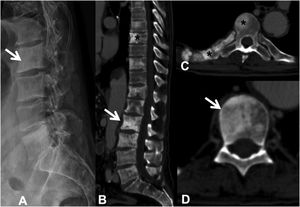
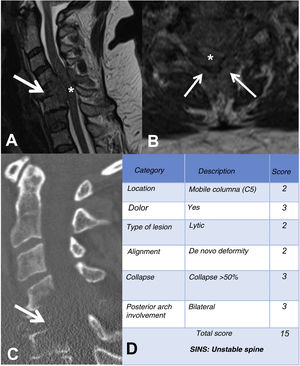
MRI is used to assess possible radicular or spinal cord compression due to vertebral body fracture or epidural soft tissue component. The Spine Instability Neoplastic Score (SINS) staging system is used to determine the stability of a pathological fracture and is very useful for surgical decision making. Mechanical instability is an indication for surgical stabilisation or percutaneous cement treatment, independent of neurological or oncological assessments. Determination of instability is almost entirely based on imaging. This classification is vital to prevent the catastrophic consequences of instability in the context of metastatic disease: severe pain, loss of function or paresis. SINS grades spinal stability by summing five radiographic and one clinical component to give a total score ranging from 0 to 18. The five radiological features include the spine location of the metastasis, spinal alignment, bone lesion quality (lytic, sclerotic, or mixed), the degree of vertical body involvement and vertical body collapse, and posterior element involvement. The only characteristic of clinical instability is the presence of movement-related pain. A high SINS score (13–18) warrants urgent surgical intervention. The indeterminate category (a score of 7–12) indicates surgical consultation as soon as possible. A score of 1–6 suggests that the spine is stable25 (Table 3 and Fig. 6).
Elements and scoring of the Spinal Instability Neoplastic Staging System (SINS).
| SINS elements | Score | |
|---|---|---|
| Location | ||
| Junctional (occipital-C2, C7-T2, T11-L1, L5-S1) | 3 | |
| Mobile spine (C3–C6, L2–L4) | 2 | |
| Semi-rigid spine (T3–T10) | 1 | |
| Rigid spine (S2–S5) | 0 | |
| Pain relief with recumbence/movement/loading | ||
| Yes | 3 | |
| No (occasional pain but not mechanical) | 1 | |
| Painless lesion | 0 | |
| Bone lesion | ||
| Lytic | 2 | |
| Mixed (lytic and blastic) | 1 | |
| Blastic | 0 | |
| Radiographic spinal alignment | ||
| Subluxation/translation | 4 | |
| De novo deformity (kyphosis/scoliosis) | 2 | |
| Normal alignment | 0 | |
| Vertebral body collapse | ||
| Collapse>50% | 3 | |
| Collapse<50% | 2 | |
| No collapse with >50% vertebral body involved | 1 | |
| None of the above | 0 | |
| Posterolateral vertebral element involvement(facet, pedicle, or joint) | ||
| Bilateral | 3 | |
| Unilateral | 1 | |
| None of the above | 0 | |
| 0–6 stable | 7–12 undetermined | 13–18 unstable |
| Consultation for surgery | ||
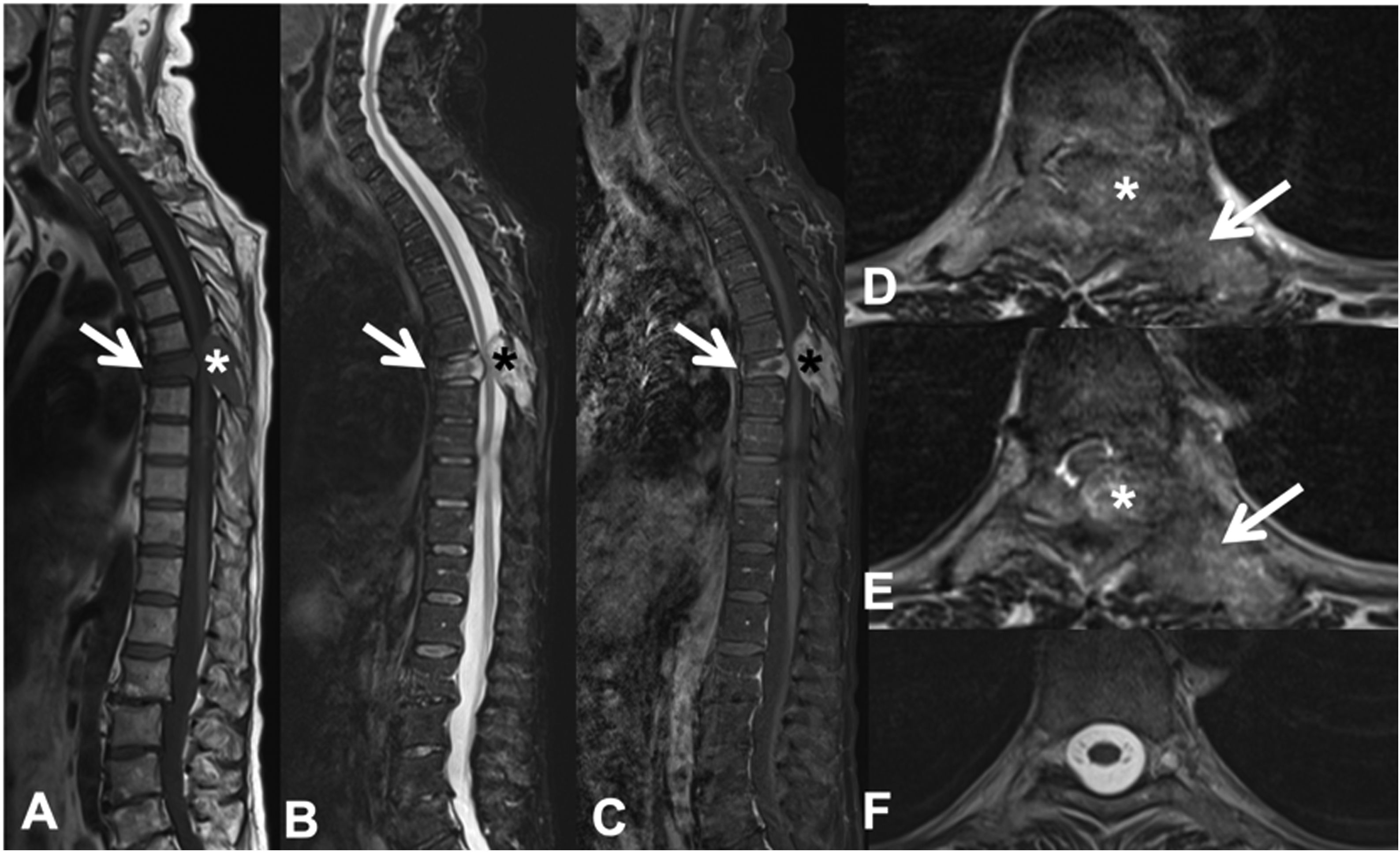
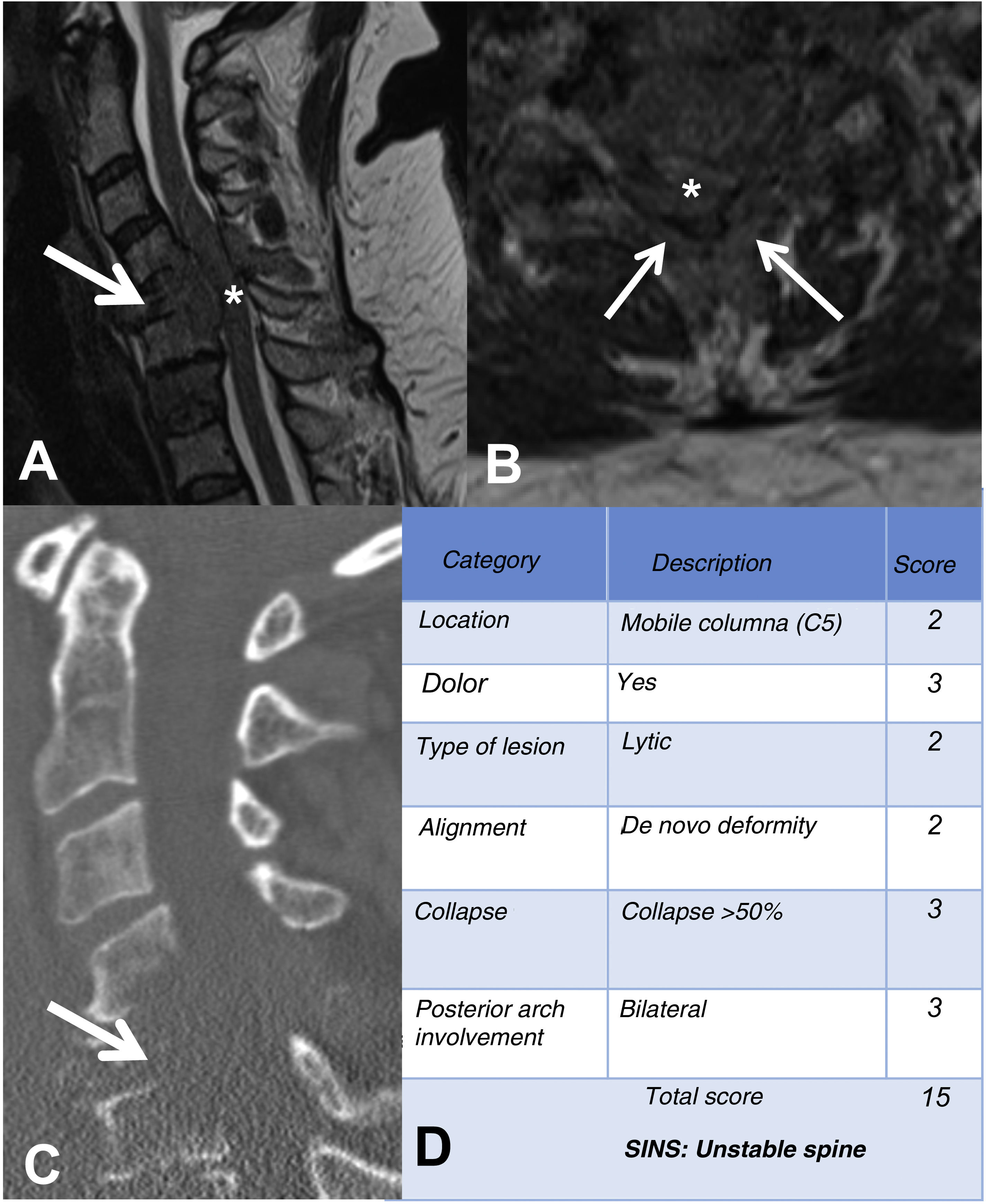
Example of the Spinal Instability Neoplastic Staging System (SINS). (A and B) T2-weighted MRI with sagittal and axial view of cervical spine, with pathological metastatic fracture of C5 with involvement of the body and posterior arch (arrow) and epidural mass (asterisk), producing spinal cord compression; (C) CT scan of cervical spine with sagittal reconstruction, showing that the metastasis is lytic; (D) table of category, description, and scoring of the lesion. CT: computed tomography; MRI: magnetic resonance imaging.
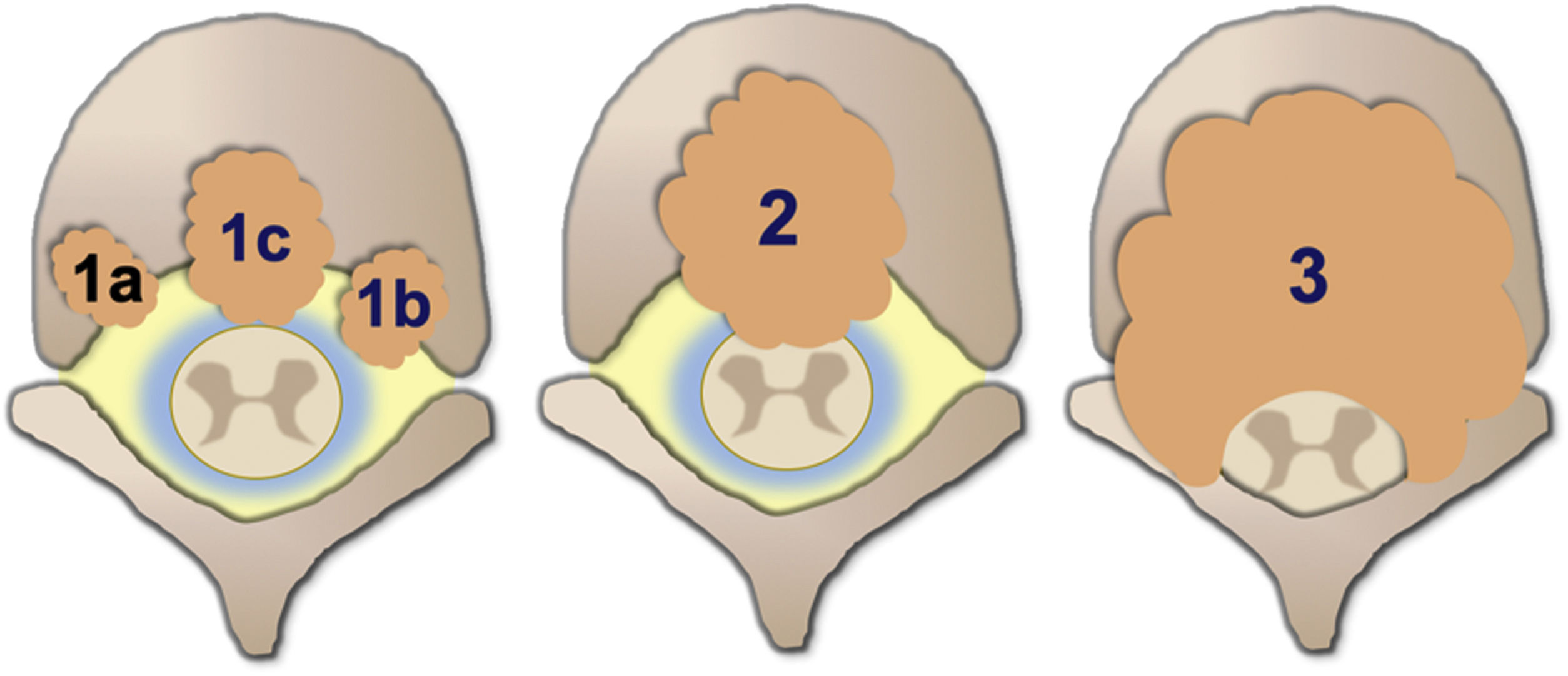
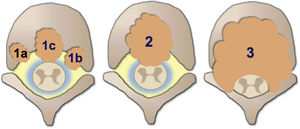
Degree of spinal cord compression is measured with the Epidural Spinal Cord Compression Scale (ESCC) by Bilsky et al.26 The ESCC is divided into low grade (0 and 1) and high grade (2 and 3). The grades are:
- -
Grade 0: bone-only disease.
- -
Grade 1a: epidural impingement, without deformation of the thecal sac.
- -
Grade 1b: deformation of the thecal sac, without spinal cord abutment.
- -
Grade 1c: deformation of the thecal sac with spinal cord abutment, but without cord compression.
- -
Grade 2: spinal cord compression, but with cerebrospinal fluid (CSF) visible around the cord.
- -
Grade 3: spinal cord compression, no CSF visible around the cord.
Neurological considerations focus on the degree of spinal cord compression. ESCC is used in conjunction with clinical assessment of myelopathy and/or radiculopathy for treatment decisions. Classification is performed on axial T2-weighted images at the site of most severe compression. If the spine is stable, radiation is considered as initial therapy of low-grade compression (ESCC grades 0, 1a, 1b, 1c). High-grade compression (grades 2 and 3) requires minimal initial surgical decompression of the epidural space, unless the tumour is very radiosensitive, or the patient cannot tolerate surgery (Fig. 7).
Illustrative diagram of the degrees of spinal cord compression with the epidural spinal compression scale (ESCC) of Bilsky et al.26
Nuclear medicine uses imaging techniques that provide functional information on the activity of tumour or metastatic cells by detecting radiation emitted by radiotracers.27,28
The most commonly used radiotracers for the detection of vertebral metastases can be classified into: osteotropic agents, which detect osteoblastic activity, such as 99mTc-methylene diphosphonate (99mTc-DMP) and 18F-sodium fluoride (18F-NaF); and oncotropic agents, which detect the metabolic activity of tumour cells, such as 18F-fluorodeoxyglucose (18F-FDG).2899mTc-DMP is the most widely used due to its effectiveness, reasonable cost, wide availability, and favourable dosimetry.2918F-FDG is a radiotracer widely used for detecting tumour and metastatic lesions.28–30
The available imaging techniques are BS, SPECT, and PET.
BS is still the most widely used nuclear medicine technique for the detection of bone metastases because it is widely available and of low cost.30,31 Using 99mTc-DMP, it detects metastatic bone deposits through increased osteoblastic activity, an indirect marker of an oncological process. Therefore, it is considered the most efficient modality for detecting metastases throughout the body.29 It is a very sensitive technique, allowing early detection of bone metastases.28 However, it has low specificity and can lead to false positives, such as trauma or infectious lesions.27,29 It can also lead to false negatives, because it is not very sensitive in identifying purely osteolytic lesions, when bone turnover is slow, or the lesion is avascular.6,29,30
SPECT detects osteoblastic activity using 99mTc-3,3-diphosphono-1,2-propanedicarboxylic acid (99mTc-DPD). But unlike BS, images are acquired in three dimensions.28
PET is used to visualise cell activity by detecting radiation emitted during positron decay. The most commonly used positron emitting radiotracers for detecting bone metastases are 18F-NaF or 18F-FDG.28 PET with 18F-FDG is more useful in distinguishing between benign and malignant bone lesions.27,30 In contrast to 99mTc-DPD BS it is much more sensitive for identifying lytic lesions and spinal metastases, it offers superior spatial resolution, while blastic metastases show lower metabolic activity and are often undetectable.27,30
The development of hybrid techniques such as SPECT/CT, PET/CT, or PET/MRI has made it possible to merge functional information from nuclear medicine with anatomical data provided by imaging techniques such as CT or MRI. Thus, diagnostic confidence increases when the bone lesion suspected as metastasis accumulates radiotracer.28
PET is the nuclear medicine technique with the best sensitivity.6 Furthermore, hybrid techniques of PET with CT and MRI are more diagnostically accurate than PET alone.28,31
In summary, nuclear medicine techniques are essential to assess the activity of vertebral metastases and are more diagnostically accurate when combined with CT and MRI.
Percutaneous treatmentPercutaneous treatment of vertebral metastases is generally of palliative intent, it could be considered a therapeutic option for pain management and only in selected patients.15,32,33
Pain caused by vertebral metastases is complex. It presents in three scenarios: the first is localised pain, resulting from periosteal involvement due to tumour expansion, the second is radicular pain, caused by compression or infiltration of the nerve root, and the third is axial pain, associated with mechanical instability or pathological fracture of the vertebral body.34 Vertebral augmentation procedures focus on the management of axial pain, or pathological fractures treated with radiotherapy or with inadequate response to systemic treatments and/or analgesia.
There are several techniques, one of the most widely used is percutaneous cementoplasty or osteoplasty. This involves the injection of polymethylmethacrylate (PMMA) into the vertebral body, in the context of a pathological fracture or to prevent fractures, it promotes consolidation of the damaged bone and significantly reduces the patient's pain. The polymerisation of PMMA produces a transient exothermic reaction (up to 80°C); this increase in temperature does not result in tumour necrosis at the bone/PMMA interface, therefore, in isolation, it has no curative intent.15,32,35 Its main indication is pain management in patients with vertebral metastatic involvement, without extension to the epidural space. It can also be performed in combination with other techniques, such as ablative techniques or radiotherapy itself.34,36
Curative treatment can be considered in selected patients with oligometastases and limited bone disease. In contrast to palliative treatment, the margins of ablation in curative treatment should extend beyond those of the tumour, as long as vital structures are not compromised.15,32 Tumour ablation techniques involve the direct application of physical or chemical agents for the local destruction of the tumour in the vertebral body, including ablation by alcohol instillation and different methods of thermoablation, such as radiofrequency, cryoablation, microwaves, and ultrasound.15,32,35 This type of procedure causes weakness of the residual bone, resulting in fragility and risk of fracture, and therefore it is usually complemented with cementoplasty or osteoplasty.22
Transarterial embolisation is another useful procedure. Its aim is to reduce the vascularisation of metastases, being as selective as possible. Its indication is to minimise the risk of bleeding when resecting hypervascular metastases using surgical techniques and also to reduce pain and the risk of spontaneous bleeding in patients who are not candidates for surgical treatment.37
Pain scales or quality of life scores are recommended to assess response to treatment. Follow-up imaging is not necessary in patients with diffuse metastatic disease, who have received palliative treatment, unless new symptoms appear. However, in those with oligometastatic disease treated with curative intent, follow-up is recommended to monitor tumour response.15,32,35
ConclusionsMRI is the best imaging modality for detecting vertebral metastases. It is important to make the differential diagnosis between vertebral fracture of osteoporotic and pathological cause. Spinal cord compression is a serious complication of metastatic disease and its assessment by imaging through objective scales is decisive for estimating spinal stability and, therefore, for establishing treatment.
Level of evidenceLevel of evidence I.
FundingNo funding was received for this study.
Conflict of interestsThe authors have no conflict of interests to declare.