Telescopic intramedullary nails (TIMN) have represented a significant advancement in limb lengthening procedures. However, their elongation capacity is limited to 5cm in the bones of patients with achondroplasia. Consequently, techniques involving TIMN reutilization have been developed. This reuse presents mechanical and safety challenges due to material fatigue and repetitive loading, which may compromise the structural integrity of the implant. This study evaluates the biomechanical performance and potential damage of a reused TIMN.
MethodsAn experimental analysis was conducted on a femoral TIMN removed after two 5-cm lengthenings in a patient with achondroplasia. The nail was measured and examined following non-destructive deconstruction, material analysis, 3D reverse engineering modeling, and finite element analysis to assess its performance under various loading conditions.
ResultsMechanical and chemical damage compromising the integrity of the nail was identified. The aged Ti6Al4V alloy was validated for its resistance to complex loads. The 3D model showed that the gear mechanism effectively transformed rotational into translational motion. Finite element analysis revealed that the safety factor reached its critical threshold at 2.44̊ and 2.25̊, indicating the nail was near its mechanical limit. The rod and guide were identified as critical components.
ConclusionsTIMN reuse should be approached with caution due to potential material fatigue. This study provides a foundation for redesigning these implants to improve their ability to withstand prolonged loading cycles.
Los clavos intramedulares telescópicos (CIMT) han supuesto un avance significativo en el alargamiento de extremidades. Sin embargo, su capacidad de alargamiento está limitada a 5cm en los huesos de pacientes con acondroplasia. Por ello, se realizan técnicas que incluyen la reutilización del CIMT. Esta reutilización plantea desafíos mecánicos y de seguridad debido a la fatiga del material y las cargas repetitivas que pueden comprometer su integridad estructural. Este estudio evalúa el comportamiento biomecánico y los posibles daños de un CIMT reutilizado.
MétodosSe realizó un análisis experimental de un CIMT de fémur retirado tras 2 alargamientos de 5cm en un paciente con acondroplasia. Se midió y evaluó el clavo tras deconstrucción no destructiva, análisis de materiales, modelado 3D por ingeniería inversa y análisis de elementos finitos para determinar su comportamiento bajo diferentes condiciones de carga.
ResultadosSe identificaron daños mecánicos y químicos que comprometieron la integridad del clavo. La aleación Ti6Al4V envejecida fue validada por su resistencia a cargas complejas. El modelado 3D mostró que el mecanismo de engranajes convertía eficazmente el movimiento rotatorio en traslacional. El análisis de elementos finitos reveló que el coeficiente de seguridad alcanzó su límite crítico a ángulos de 2,44 y 2,25°, lo que evidencia que el clavo estaba cerca de su límite de resistencia. El vástago y la guía fueron componentes críticos.
ConclusionesLa reutilización de los CIMT debe realizarse con precaución debido a la posible fatiga del material. Este estudio proporciona una base para el rediseño de estos implantes, mejorando su capacidad para soportar ciclos prolongados de carga.
Achondroplasia, the most common form of skeletal dysplasia, is characterised by disproportionately short stature due to a mutation in the fibroblast growth factor receptor 3 gene.1,2 This mutation impairs endochondral ossification, resulting in a phenotype that includes rhizomelic shortening of the limbs, macrocephaly, and joint laxity, as well as clinical complications such as sleep apnoea and spinal canal stenosis.3
The average adult height for people with achondroplasia is 130cm for males and 124cm for females.4 This short stature has a significant impact on daily life, limiting the ability to overcome physical and architectural barriers and affecting social integration and self-esteem.5 Despite therapeutic advances, such as the use of vosoritide and other therapeutic strategies under investigation to modulate the fibroblast growth factor receptor 3 signalling pathway, such as infigratinib, navepegritide, and TYRA-300, their high cost and lack of long-term data limit their widespread clinical use.6 Therefore, limb lengthening via distraction osteogenesis remains the primary treatment option to increase stature and improve functionality in these patients. Traditionally, external fixators were used for this purpose7–9; however, in recent years, telescopic intramedullary nails (TIMN) have emerged as a more effective and better-tolerated alternative.10–14
However, the lengthening capacity of TIMN in short bones is limited to 5cm per nail, which is insufficient for patients with achondroplasia. To overcome this, TIMN have been reused in sequential lengthening procedures, enabling a cumulative elongation of up to 10cm.15–17 The first series of cases reusing the same TIMN in patients with achondroplasia was published in 2022, achieving length gains exceeding 10 cm per bone.18
Although this strategy partially addresses the issue of limited elongation capacity, reusing a TIMN originally designed for single use raises concerns regarding its mechanical integrity and sustained performance throughout multiple lengthening cycles.19,20 Sequential procedures prolong distraction time, potentially increasing implant load and the risk of material fatigue. Recent studies have documented failures in magnetic intramedullary nails subjected to prolonged lengthening,21,22 highlighting the need to evaluate their structural behaviour in these scenarios.
This study aims to analyse the biomechanical performance of a femoral TIMN explanted after two consecutive lengthening procedures in a patient with achondroplasia. The objective is to identify any alterations in the implant, and determine critical zones of wear or fatigue under controlled experimental conditions, in order to propose improvements to enhance the durability, biomechanical performance, and safety of TIMNs in sequential use.
Material and methodsThis experimental study was conducted at the Higher Technical School of Engineering and Industrial Design of the Polytechnic University of Madrid between October and December 2023. It involved the analysis of a femoral TIMN that had been removed from a patient with achondroplasia treated at the Hospital Infantil Universitario Niño Jesús. The implant had been reused for two consecutive 5 cm lengthening procedures using a sequential distraction technique, as previously described in the literature.18 Unlike conventional reuse, the implant was not removed or replaced in this case, but rather retracted using the magnetic mechanism to allow a second lengthening phase without interrupting treatment.
Measurement and validation processInitial measurements of the TIMN were taken in detail, recording its geometry and singular features such as bevels, ribs, and geometric transitions. A high-precision digital caliper (tolerance: hundredths of a millimetre) and a tailor's magnifying glass with neck support and fixators were used. The nail was then deconstructed non-destructively via a controlled longitudinal cut from the proximal to the distal end using a rotary cutter, preserving the structural integrity of the components. Additional transverse sections were performed in specific regions to release internal elements without damaging their configuration. The TIMN was divided into functional subunits: the guide, magnet assembly, gear box, rod, shaft, and crown.
To validate the measurements, each part was measured ten times, and the mean value was used as the theoretical reference. AutoCAD 2024 and Autodesk Inventor 2023 were employed to analyse and reconstruct small or complex parts in detail (Fig. 1).
Analysis of parts using AutoCAD 2024 software. (A) Measurement of the magnet. (B) Toothed geometry of the gear box, showing the use of threads. (C) Enlarged comparison of the gear box with 36 teeth. (D) Image of the bearing assembly with 7 bearings measured with Autodesk Inventor. (E) Screenshot of the dimensional measurement of the bearing assembly.
The nail's material was analysed using Ansys Granta EduPack 2023R software. A titanium–aluminium–vanadium (Ti6Al4V) alloy was selected for its biocompatibility and mechanical properties. Characteristics such as tensile strength, elastic limit and biological compatibility were evaluated to ensure that the material could withstand the loads associated with bone elongation without compromising its integrity.
Three-dimensional modellingA full 3D model of the TIMN was developed by reverse engineering using AutoCAD and Autodesk Inventor, reconstructing each component with high fidelity. This allowed visualisation of internal interactions and confirmation of correct mechanical function throughout the lengthening cycle. Digital reconstruction of the nail was then used to evaluate possible points of structural stress, facilitating subsequent biomechanical simulation.
Finite element analysisStructural analysis was performed using Autodesk Inventor Professional 2023, applying a finite element model (FEM). We simulated the loads that the nail would withstand during lengthening in a 12-year-old patient with achondroplasia, based on representative anthropometric data for this population.23 The analysis included two scenarios: one representing the beginning of the lengthening treatment and the other 12 months into the process. This enabled the behaviour of the nail under different load conditions to be evaluated, identifying the points of greatest stress, deformation, and risk of structural failure.
The simulations were carried out under ideal conditions of fit between the nail and the femur, with variations in the angles of inclination to simulate different load situations in the axial and torsional directions, caccounting for patient body weight and functional demands during treatment. Additionally, repetitive load simulations were modelled to evaluate the potential for fatigue failure over time. With this implant, it is important to note that loading is delayed until three or four cortices have consolidated. This differs biomechanically from other elongation methods, such as external fixation or a combination of external fixation and a standard intramedullary nail, where early loading is an additional factor in evaluating implant strength. The critical point was defined as the moment when the safety factor reached λ = 1, indicating the threshold for structural failure. Principal stresses and strains were evaluated in both scenarios to identify key areas of vulnerability (Fig. 2).
ResultsAnalysis of measurements and external geometryDuring the opening of the nails, mechanical damage was observed, including thread marks from the rod onto the guide, as well as signs of possible chemical deterioration likely due to prolonged exposure to organic fluids. These alterations affected critical regions of the implant and may compromise its overall structural integrity (Fig. 3).
Material analysisDetailed analysis of the mechanical properties, including von Mises stress, identified aged Ti6Al4V as the most suitable alloy for this application, due to its high resistance to complex mechanical loading and compliance with biomedical standards (Fig. 4). The alloy was validated using Ansys Granta EduPack 2023R software, confirming its tensile strength and long-term suitability for implantable medical use.
Three-dimensional model analysisThe reconstructed 3D model demonstrated that the internal mechanism, comprising a magnet-driven gear train, allows effective transformation of rotational into translational motion, thus enabling the intended distraction function of the nail (Fig. 5).
(A) Overview of 3D model of the initial nail reconstructed using reverse engineering. (B) Cross-section of the nail showing the internal layout. (C) Detail of the rod without the guide wire, highlighting the interaction between the magnet, the gears, and the rod. (D) Gear box attached to the magnet. (E) Gear train of the 1/64 reduction mechanism, geared from the magnet in the distal part to the nail in the proximal part, effectively transmitting movement.
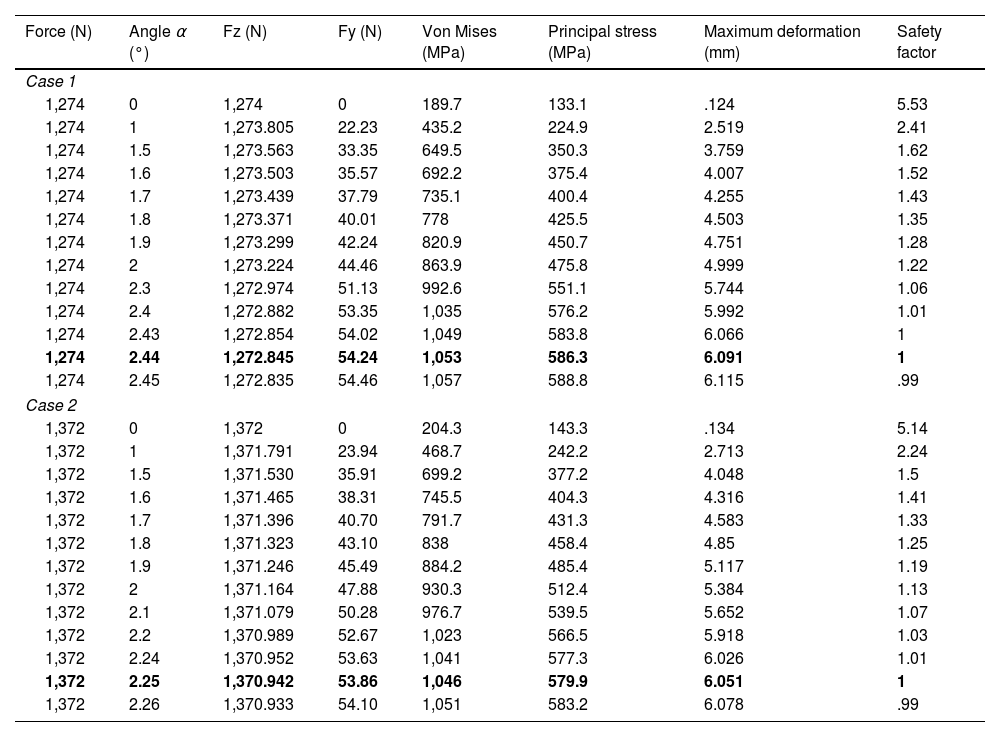
FEM analysis revealed that the safety factor reached the critical threshold of 1 at inclination angles of 2.44° and 2.25° for cases 1 and 2, respectively. This indicates that the nail approaches its structural resistance limit under physiological loading conditions. These results suggest a significant risk of fatigue failure if the implant is subjected to additional loads (Table 1). As the inclination angle increased, the stresses in the material rose progressively, reaching critical levels of deformation and von Mises stress.
Detailed results of the FEM analysis for cases 1 and 2, highlighting the forces, stresses, and deformations experienced under different angles of inclination.
| Force (N) | Angle α (°) | Fz (N) | Fy (N) | Von Mises (MPa) | Principal stress (MPa) | Maximum deformation (mm) | Safety factor |
|---|---|---|---|---|---|---|---|
| Case 1 | |||||||
| 1,274 | 0 | 1,274 | 0 | 189.7 | 133.1 | .124 | 5.53 |
| 1,274 | 1 | 1,273.805 | 22.23 | 435.2 | 224.9 | 2.519 | 2.41 |
| 1,274 | 1.5 | 1,273.563 | 33.35 | 649.5 | 350.3 | 3.759 | 1.62 |
| 1,274 | 1.6 | 1,273.503 | 35.57 | 692.2 | 375.4 | 4.007 | 1.52 |
| 1,274 | 1.7 | 1,273.439 | 37.79 | 735.1 | 400.4 | 4.255 | 1.43 |
| 1,274 | 1.8 | 1,273.371 | 40.01 | 778 | 425.5 | 4.503 | 1.35 |
| 1,274 | 1.9 | 1,273.299 | 42.24 | 820.9 | 450.7 | 4.751 | 1.28 |
| 1,274 | 2 | 1,273.224 | 44.46 | 863.9 | 475.8 | 4.999 | 1.22 |
| 1,274 | 2.3 | 1,272.974 | 51.13 | 992.6 | 551.1 | 5.744 | 1.06 |
| 1,274 | 2.4 | 1,272.882 | 53.35 | 1,035 | 576.2 | 5.992 | 1.01 |
| 1,274 | 2.43 | 1,272.854 | 54.02 | 1,049 | 583.8 | 6.066 | 1 |
| 1,274 | 2.44 | 1,272.845 | 54.24 | 1,053 | 586.3 | 6.091 | 1 |
| 1,274 | 2.45 | 1,272.835 | 54.46 | 1,057 | 588.8 | 6.115 | .99 |
| Case 2 | |||||||
| 1,372 | 0 | 1,372 | 0 | 204.3 | 143.3 | .134 | 5.14 |
| 1,372 | 1 | 1,371.791 | 23.94 | 468.7 | 242.2 | 2.713 | 2.24 |
| 1,372 | 1.5 | 1,371.530 | 35.91 | 699.2 | 377.2 | 4.048 | 1.5 |
| 1,372 | 1.6 | 1,371.465 | 38.31 | 745.5 | 404.3 | 4.316 | 1.41 |
| 1,372 | 1.7 | 1,371.396 | 40.70 | 791.7 | 431.3 | 4.583 | 1.33 |
| 1,372 | 1.8 | 1,371.323 | 43.10 | 838 | 458.4 | 4.85 | 1.25 |
| 1,372 | 1.9 | 1,371.246 | 45.49 | 884.2 | 485.4 | 5.117 | 1.19 |
| 1,372 | 2 | 1,371.164 | 47.88 | 930.3 | 512.4 | 5.384 | 1.13 |
| 1,372 | 2.1 | 1,371.079 | 50.28 | 976.7 | 539.5 | 5.652 | 1.07 |
| 1,372 | 2.2 | 1,370.989 | 52.67 | 1,023 | 566.5 | 5.918 | 1.03 |
| 1,372 | 2.24 | 1,370.952 | 53.63 | 1,041 | 577.3 | 6.026 | 1.01 |
| 1,372 | 2.25 | 1,370.942 | 53.86 | 1,046 | 579.9 | 6.051 | 1 |
| 1,372 | 2.26 | 1,370.933 | 54.10 | 1,051 | 583.2 | 6.078 | .99 |
Fz (N) and Fy (N) represent the forces acting in the “z” and “y” directions, respectively.
The limit cases for which the safety index is the minimum acceptable are shown in bold.
Table 2 summarises the results of the FEM analysis and compares the structural behaviour under different inclination angles in both cases.
The analysis relied on von Mises and principal stress concepts for mechanical validity: von Mises stress provides an integrated measure of the stress state to anticipate the onset of plastic deformation, while principal stresses help identify specific vectors of failure under complex load configurations.
The design of the implant offers adequate mechanical stability for the distraction process, particularly under compressive loads. However, both the guide wire and the rod were identified as critical components for the stability of the system, with the rod being particularly vulnerable to failure under prolonged stress. FEM simulations showed that the nail, rod, and gear train distribute stresses efficiently, allowing the conversion of rotational motion into translational motion without compromising the functionality of the system.
DiscussionThe results of this study confirm that while the reuse of TIMN can be a feasible option under specific conditions, it also entails limitations. A central concern is the risk of mechanical failure due to material fatigue and repetitive loading, which underscores the need for safer strategies to optimise extended use.
Although TIMN are an attractive alternative in terms of reducing costs and minimising surgical trauma, the manufacturer's technical data sheet does not contemplate reuse, as the devices are designed for single-lengthening procedures. Reuse aims to maximise elongation without reinsertion of a new implant, thereby reducing the surgical risks associated with re-nailing. However, this strategy poses risks, especially with regard to the mechanical deterioration of the nail and its elongation system, as these devices have not been designed for multiple reuse cycles.
From a biomechanical perspective, the resistance of TIMN is determined by their geometry and the materials used, which influences their ability to withstand prolonged loads. Our findings indicate that, while TIMN tolerate substantial loading, their performance approaches structural limits under sequential use. While FEM analysis did not reveal any immediate failures, simulations showed that the accumulated stress at critical points, such as the rod and the 11° angle, could compromise the structural integrity of the implant under repetitive loading conditions. The telescopic design, with its moving internal components, introduces additional vulnerability to torsional and bending forces, which can accelerate material fatigue compared to conventional fracture nails. Previous studies, such as that by Panagiotopoulou et al., have demonstrated the vulnerability of telescopic joints to torsional and bending forces, thereby increasing the risk of fatigue and eventual material failure.19 This risk is accentuated in reused nails, as additional elongation cycles generate a cumulative increase in mechanical stress, which can accelerate implant degradation.19 Previous clinical series on reused nails in achondroplasia, reported no fractures in 26 reused devices. 18 However, other studies do report nail fractures under excessive loads or after reuse, such as the PRECICE® nail that broke after being reactivated for an elongation procedure, highlighting the risk of material fatigue and accumulated mechanical stress, especially when the recommended weight load restrictions are not strictly adhered to.22
Regarding reactivation, the 3D reconstruction of all meshed components demonstrated the correct transmission of rotational to translational motion, with no faults detected in the nail's internal mechanism. However, previous research has shown mixed results regarding the reuse of TIMN. Georgiadis et al. reported that only 58% of reused nails were able to complete a second lengthening without replacement, while 42% of cases resulted in failure or complications requiring implant replacement.22 Eltayeby et al. analysed 102 reused PRECICE® nails and found that, after months of inactivity, 84.3% were successfully reactivated, although some failed due deformities in the nail or defects in the telescopic mechanism. This damage is especially common after the nail has been fully deployed, as this prevents it from retracting and functioning correctly in subsequent procedures.20 Finally, Alonso-Hernández et al., in their series of 26 nails reused for a second 5cm lengthening in patients with achondroplasia, demonstrated success rates of over 90% (24 out of 26 nails), showing that reactivation may be feasible under certain controlled conditions.18
Another relevant concern in the reuse of intramedullary nails is the internal degradation of their mechanism, which can lead to structural failure and the potential release of metal particles.19 While newer TIMN models have shown a lower incidence of this issue, caution is still warranted when considering their reuse. In our analysis, we identified obvious mechanical damage, such as wear marks on the rod's threading at the guide interface, and signs of possible chemical damage from prolonged exposure to organic fluids. These findings suggest that TIMN reuse could increase the risk of metal particle release into the bloodstream. This phenomenon has been documented in other magnetic devices such as growing rods used in early-onset scoliosis. Although this phenomenon appears less frequent in TIMN due to their different structure and usage patterns, no studies to date have evaluated systemic metal levels in patients with reused nails. This represents an important avenue for future research.
This study has several limitations. First, the analysis was based on a single nail, which may limit the generalisability of the findings. Second, the absence of a control group with nails used for a single 5 cm lengthening precludes direct biomechanical comparisons between standard and sequential elongation. Future studies should incorporate such comparisons to more accurately assess the mechanical consequences of reuse. Additionally, heterogeneity in reuse protocols across institutions could affect the structural integrity of reused implants and limit the external validity of our results. Furthermore, FEM simulations were conducted under idealised mechanical conditions, without accounting for biological interactions with surrounding tissues or the corrosive effects of body fluids. These aspects have been reported in post-retrieval studies of implants. Finally, the lack of long-term clinical follow-up data on reused TIMN restricts our understanding of their durability and safety profile.
Despite these limitations, this study offers meaningful contributions by integrating reverse engineering, 3D modelling, and FEM simulations to biomechanically characterise a reused TIMN. This approach represents a valuable first step toward understanding the mechanical risks of sequential elongation and informs future efforts to design more robust and reusable intramedullary devices.
ConclusionsIn conclusion, the present study confirms that although TIMN provide clinical advantages, their reuse demands careful evaluation. Repeated loads during elongation may compromise the structural integrity of the implant, increasing the risk of mechanical failure. Furthermore, these findings provide a foundation for redesigning TIMNs to enhance their robustness and ability to withstand prolonged loading cycles without compromising their functionality, thus making reuse a safer and more viable option.
Level of evidenceLevel of evidence iv.
CRediT authorship contribution statementAll the authors made substantial contributions to the conception or design of the work, or to the acquisition, analysis, or interpretation of the data in the work; drafting of the work or critical revision of the work for important intellectual content; final approval of the version to be published; and agree to be accountable for all aspects of the work, ensuring that questions regarding the accuracy or integrity of any part of the work are investigated and appropriately resolved.
Informed consentInformed consent was not required, as the study did not involve human subjects or the use of personally identifiable information, but was limited to the analysis of a reused medical device.
Ethical approvalThis study did not require approval by an ethics committee, as it is an experimental analysis of a telescopic intramedullary nail removed after clinical use, without directly involving patients or the use of personal data.
FundingThis project received support from the SECOT Foundation and the Alonso Family Foundation for its development.
Conflict of interestThe authors have no conflict of interests to declare.
We sincerely thank the SECOT Foundation and the Alonso Family Foundation for their help and support in conducting this study.