Diabetic foot (DF) is a common and serious complication of diabetes mellitus (DM), especially in patients with chronic kidney disease (CKD) undergoing renal replacement therapy (RRT). This study aimed to assess the prevalence of DF and associated conditions in DM patients receiving RRT at a tertiary care hospital in Argentina.
Materials and methodsWe conducted a cross-sectional observational study between December 2022 and September 2024. A total of 54 patients with type 1 or type 2 DM undergoing either hemodialysis (HD) or peritoneal dialysis (PD) were included. History of DF, active or pre-ulcerative lesions, neuropathy, peripheral vascular disease, and associated risk factors were evaluated through physical examination and medical record review.
ResultsDF was present in 40.7% of patients, with a higher proportion in HD (48.6%) compared to PD (26.3%). Pre-ulcerative lesions were found in 61.1%, and active ulcers in 9.3%. A history of amputation was reported in 31.4% of cases. Diabetic neuropathy (87%) and peripheral vascular disease (81.5%), both closely related to DF development, were key findings. Significant differences were observed in smoking (42.1% PD vs. 11.4% HD, p=0.016), which may impair microcirculation, and obesity (63.2% PD vs. 25.7% HD, p=0.016), which increases plantar pressure and contributes to foot deformities.
ConclusionsDM patients on RRT have a high prevalence of DF and related risk factors. Early detection and multidisciplinary follow-up are essential to prevent complications such as ulcers and amputations.
El pie diabético (PD) es una complicación frecuente y grave de la diabetes mellitus (DM), particularmente en pacientes con enfermedad renal crónica (ERC) en terapia sustitutiva renal (TSR). Este estudio evalúa la prevalencia de PD y condiciones asociadas en pacientes con DM bajo TSR en un hospital de tercer nivel en Argentina.
Material y métodosSe realizó un estudio observacional, transversal entre diciembre de 2022 y septiembre de 2024. Se incluyeron 54 pacientes con DM tipo 1 o 2 en tratamiento con hemodiálisis (HD) o diálisis peritoneal (DP). Se evaluaron antecedentes de PD, lesiones activas o preulcerativas, neuropatía, enfermedad vascular periférica y factores de riesgo asociados mediante examen físico y revisión de la historia clínica.
ResultadosLa prevalencia de PD fue del 40,7%, con mayor proporción en HD (48,6%) frente a DP (26,3%). Se observaron lesiones preulcerativas en el 61,1% de los pacientes y lesiones activas en el 9,3%. El 31,4% tenía antecedentes de amputaciones. La neuropatía diabética (87%) y la enfermedad vascular periférica (81,5%), condiciones estrechamente relacionadas con la aparición del PD, fueron hallazgos clave. Asimismo, se observaron diferencias significativas en factores de riesgo como el tabaquismo (42,1% DP vs. 11,4% HD, p=0,016), que puede deteriorar la microcirculación, y la obesidad (63,2% DP vs. 25,7% HD, p=0,016), la cual incrementa la presión plantar y favorece las deformidades del pie.
ConclusionesLos pacientes con DM en TSR presentan una alta prevalencia de PD y factores predisponentes. La detección temprana y el seguimiento multidisciplinario son fundamentales para prevenir complicaciones graves como úlceras y amputaciones.
Diabetes mellitus (DM), particularly type 2, is one of the most prevalent chronic diseases globally and is a significant cause of complications that affect patients’ quality of life, especially in those with advanced chronic conditions. According to estimates from the International Diabetes Federation, approximately 463 million people have diabetes, a figure expected to increase to 700 million by the year 2045.1 Among the most challenging complications with high clinical impact is diabetic foot (DF), which can lead to severe ulcerations and the need for amputations. DF is defined as infection, ulceration, or destruction of deep tissues in the foot, associated with diabetic neuropathy and peripheral arterial disease in the lower limbs, potentially resulting in limb amputations.2 The social and economic burden of this condition is considerable, and it is estimated that up to 25% of patients with diabetes will develop a foot ulcer at some point in their lives.1 In patients with DM who have advanced chronic kidney disease (CKD) and require renal replacement therapy (RRT), the risk of foot complications increases significantly.3 Various studies have demonstrated that dialysis, whether hemodialysis (HD) or peritoneal dialysis (PD), constitutes an independent risk factor for the development of foot ulcers and other lesions due to factors such as advanced neuropathy, peripheral arterial disease, and immune alterations inherent to these patients.3–5 It has been reported that approximately 20% of diabetic patients on dialysis develop foot ulcers during the first year of treatment, with an annual amputation rate of 4% in this population, a figure that exceeds that observed in other groups of diabetic patients.6
The objective of this study is to assess the prevalence of DF and other associated conditions in patients with DM undergoing RRT, including both HD and PD, at a tertiary-level hospital in Argentina.
Materials and methodsA cross-sectional observational study was conducted between December 2022 and September 2024. All patients over 18 years of age with a confirmed diagnosis of type 1 or 2 DM and CKD under active RRT with HD or PD at a tertiary-level hospital in the Autonomous City of Buenos Aires, Argentina, were included. Patients were recruited from the nephrology center during their routine clinical visits at the hospital's Nephrology Unit. Patients were considered to have DM if they had ever been diagnosed with the disease or, alternatively, if they were receiving any hypoglycemic agent. All participants were required to have a complete electronic medical record and to have voluntarily agreed to undergo a physical foot examination.
Patients were excluded if they had an incomplete medical record, were not undergoing RRT properly or were receiving it at another center, or if they did not agree to participate in the physical examination or did not have a recent examination recorded in their medical record.
Patients were classified into two groups according to the type of RRT received – either hemodialysis or peritoneal dialysis. Data collection was performed using the electronic medical record in addition to a clinical evaluation based on the “Foot assessment form for patients with diabetes” from the 2021 guidelines of the Argentine Ministry of Health,7 along with a physical foot examination for all diabetic patients undergoing RRT.
For each participant, the following variables were collected: age, gender, type and duration of DM, most recent glycated hemoglobin (HbA1c) value, type and duration of RRT (i.e., HD or PD), consultations within the past year with the orthopedic diabetic foot service and the multidisciplinary metabolism team, fundus examination within the past year, smoking, obesity, neuropathy, and peripheral vascular disease.
In addition, patients were questioned about previous history of foot ulcers and were evaluated following the current consensus of the International Working Group on the Diabetic Foot (IWGDF 2023 update).8 All patients underwent a physical foot examination to identify current signs of infection, ulceration, or destruction of foot tissues, previous lower limb amputations (minor or major), pre-ulcerative lesions, onychodystrophy, and foot deformities. Foot deformity was defined according to the IWGDF recommendations,9 as structural abnormalities of the foot such as hammer toes, mallet toes, claw toes, hallux valgus, prominent metatarsal heads, residual neuro-osteoarthropathy, amputations, or other foot surgeries.
The presence or absence of peripheral vascular disease was determined based on lower limb Doppler ultrasound, in which the presence of atherosclerotic plaques and the level of vascular obstruction were evaluated.
Additionally, all patients who attended the DF outpatient clinic of the Foot and Ankle Section of the Orthopedics and Traumatology Department and met the inclusion criteria were recorded. Those who were not under regular follow-up with the service were given a practical preventive care guide and were scheduled for outpatient follow-up visits.
Global results regarding DF prevalence were obtained for all patients, and comparative analysis of variables between both groups was performed.
The Ethics Committee for Research Protocols of our hospital approved the study protocol.
Statistical analysisIn the descriptive analysis, quantitative data were expressed as mean and standard deviation (SD) or as median and interquartile range (IQR 25–75), depending on their distribution, while qualitative variables were expressed as absolute and relative frequencies (percentages).
Comparisons between groups according to the type of dialysis were performed using the t-test or Wilcoxon test for quantitative variables, depending on their distribution, and using the chi-square or Fisher's exact test for qualitative variables, depending on assumptions. A significance level of less than 5% was considered statistically significant. Statistical analysis was performed using STATA software version 16.0.
ResultsDuring the study period, a total of 60 patients were analyzed. Six patients were excluded: 2 due to death, 3 due to transfer to another dialysis center, and 1 due to having undergone transplantation. Finally, a total of 54 patients with DM undergoing renal replacement therapy were included: 35 (64.8%) on HD and 19 (35.2%) on PD. The mean age was 65.9 years (range 34–88), and 70.3% of the study population were male (38/54). All were type 2 diabetic patients except for one who had type 1 DM. The median age at diabetes diagnosis was 18 years (IQR: 11–23).
Fourteen patients (25.9%) had follow-up in the past year with the Orthopedics Service, and a larger number, 38 patients (70.4%), had follow-up with the multidisciplinary metabolism team.
Among the comorbidities evaluated, 47 patients (87%) had diabetic neuropathy and 44 (81.5%) had peripheral vascular disease (PVD). The prevalence of neuropathy was slightly higher in the PD group, with 17 out of 19 patients (89.5%), compared to 30 out of 35 (85.7%) in HD. PVD was more frequent among patients on HD, with 33 out of 35 (94.7%), while in PD it was observed in 14 out of 19 (74.3%). None of these differences were statistically significant. These conditions were highly prevalent in both groups; however, no direct statistical association with the presence of diabetic foot was established in this study.
Other relevant risk factors were identified, such as smoking and obesity, which showed statistically significant differences between groups. The prevalence of smoking was significantly higher in the PD group compared to HD, reported in 12 of the 54 patients (22.2%): 8 out of 19 in PD (42.1%) and 4 out of 35 in HD (11.4%) (p=0.016). Obesity was present in 21 patients (38.9%): 12 out of 19 in PD (63.2%) and 9 out of 35 in HD (25.7%), showing a higher prevalence in the PD group with a statistically significant difference (p=0.016). Regarding glycated hemoglobin (HbA1c), mean values were similar between groups (HD: 7.28; PD: 7.22; p=0.908).
For interdisciplinary follow-up, 38 patients (70.4%) were evaluated by the metabolism team during the past year: 17 out of 19 in PD (89.5%) and 21 out of 35 in HD (60.0%) (p=0.051). Follow-up by the Orthopedics Service was documented in 14 patients (25.9%): 3 out of 19 in PD (15.8%) and 11 out of 35 in HD (31.4%) (p=0.331). See Table 1.
Demographic data and comorbidities.
| Variable | All(N=54) | Peritoneal dialysis(N=19) | Hemodialysis(N=35) | p-Value |
|---|---|---|---|---|
| Years on dialysis – median (IQR) | 2.00 [1.00;4.00] | 2.00 [1.50;3.00] | 2.00 [1.00;5.00] | 0.509 |
| Years with diabetes – median (IQR) | 18.0 [11.0;23.0] | 18.0 [12.0;30.0] | 17.0 [11.0;23.0] | 0.341 |
| Last year follow-up with orthopedics – n (%) | 14 (25.9%) | 3 (15.8%) | 11 (31.4%) | 0.728 |
| Under orthopedics follow-up – n (%) | 14 (25.9%) | 3 (15.8%) | 11 (31.4%) | 0.331 |
| Last year follow-up with metabolism clinic – n (%) | 38 (70.4%) | 17 (89.5%) | 21 (60.0%) | 0.051 |
| Glycated hemoglobin – mean (SD) | 5.51 (3.44) | 7.22 (1.62) | 7.28 (1.70) | 0.908 |
| Eye examination performed – n (%) | 31 (57.4%) | 15 (78.9%) | 16 (45.7%) | 0.038 |
| Smoking – n (%) | 12 (22.2%) | 8 (42.1%) | 4 (11.4%) | 0.016 |
| Obesity – n (%) | 21 (38.9%) | 12 (63.2%) | 9 (25.7%) | 0.016 |
| Diabetic neuropathy – n (%) | 47 (87.0%) | 17 (89.5%) | 30 (85.7%) | 1 |
| Peripheral vascular disease – n (%) | 44 (81.5%) | 18 (94.7%) | 26 (74.3%) | 0.08 |
| Posterior tibial pulse – n (%) | 37 (68.5%) | 10 (52.6%) | 27 (77.1%) | 0.122 |
| Anterior tibial pulse – n (%) | 40 (74.1%) | 11 (57.9%) | 29 (82.9%) | 0.058 |
The overall prevalence of DF in the studied population was 40.7% (22/54), being higher among patients on hemodialysis (48.6%) compared to those on peritoneal dialysis (26.3%), although this difference did not reach statistical significance (p=0.194).
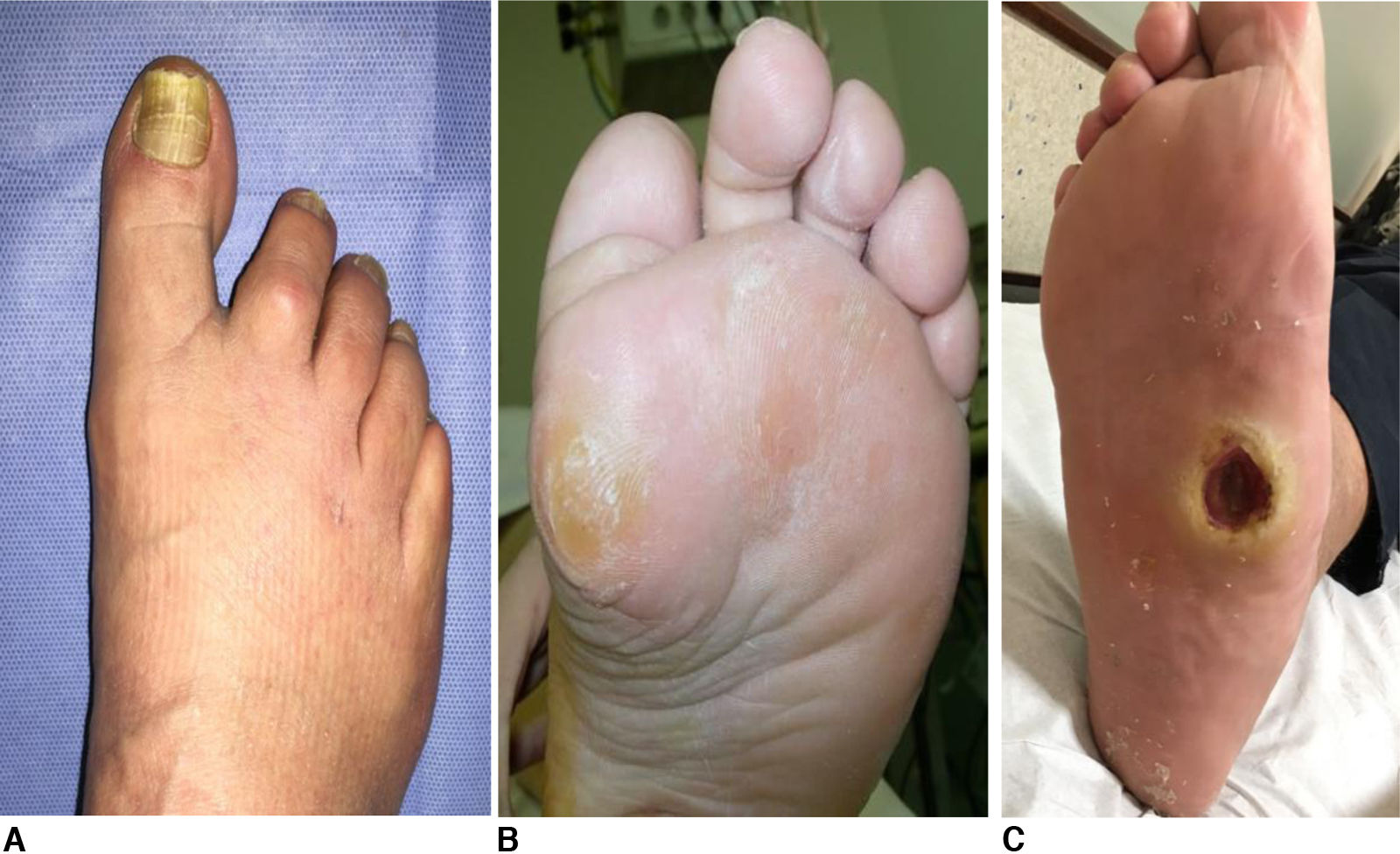
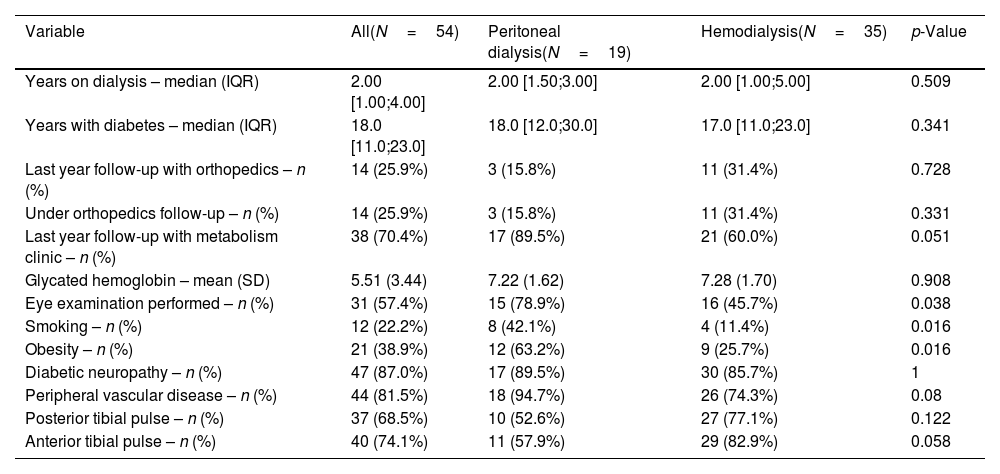
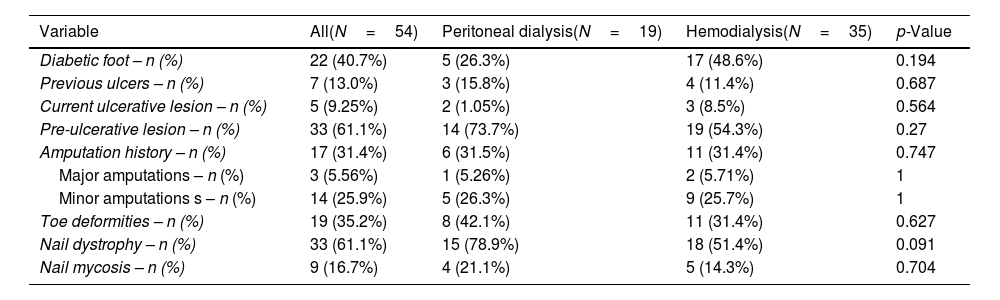
Out of the total sample, 5 of the 54 patients (9.2%) presented ulcerative lesions at the time of the examination with no prior follow-up, and 33 patients (61.1%) presented pre-ulcerative lesions on physical foot examination, with no significant differences between the HD and PD groups (54.3% and 73.7%, respectively; p=0.27). See Fig. 1.
Regarding the history of amputations, 31.4% (17/54) of the patients had undergone previous amputations, with a similar distribution between the groups (HD: 31.4%; PD: 31.5%). Of these, three patients (5.6%) had a history of major amputations (below the knee), while the remaining 14 patients had minor amputations. However, no statistically significant differences were found between the two groups.
See Table 2.
Diabetic foot prevalence and physical examination.
| Variable | All(N=54) | Peritoneal dialysis(N=19) | Hemodialysis(N=35) | p-Value |
|---|---|---|---|---|
| Diabetic foot – n (%) | 22 (40.7%) | 5 (26.3%) | 17 (48.6%) | 0.194 |
| Previous ulcers – n (%) | 7 (13.0%) | 3 (15.8%) | 4 (11.4%) | 0.687 |
| Current ulcerative lesion – n (%) | 5 (9.25%) | 2 (1.05%) | 3 (8.5%) | 0.564 |
| Pre-ulcerative lesion – n (%) | 33 (61.1%) | 14 (73.7%) | 19 (54.3%) | 0.27 |
| Amputation history – n (%) | 17 (31.4%) | 6 (31.5%) | 11 (31.4%) | 0.747 |
| Major amputations – n (%) | 3 (5.56%) | 1 (5.26%) | 2 (5.71%) | 1 |
| Minor amputations s – n (%) | 14 (25.9%) | 5 (26.3%) | 9 (25.7%) | 1 |
| Toe deformities – n (%) | 19 (35.2%) | 8 (42.1%) | 11 (31.4%) | 0.627 |
| Nail dystrophy – n (%) | 33 (61.1%) | 15 (78.9%) | 18 (51.4%) | 0.091 |
| Nail mycosis – n (%) | 9 (16.7%) | 4 (21.1%) | 5 (14.3%) | 0.704 |
This cross-sectional study addresses the prevalence and characteristics of DF in patients with DM undergoing RRT at a tertiary care hospital in Argentina. With a DF prevalence of 40.7%, our results underscore the high risk of complications in this population and reinforce existing evidence that dialysis treatment, both HD and PD, increases susceptibility to DF development in patients with advanced CKD. Comparatively, previous studies such as that by Dòria et al.4 in a similar population in Spain reported a DF prevalence of 47.6% in patients on RRT, which shows significant alignment with our findings and suggests that the risk of DF in these patients is a consistent phenomenon across various regions and healthcare settings.
Although our study did not include a control group of patients with DM not on RRT, previous studies have demonstrated that the prevalence of DF is significantly lower in that population. For example, Apelqvist et al.10 estimated that up to 25% of diabetic patients develop a foot ulcer during their lifetime, whereas in our sample of patients on RRT, the prevalence was 40.7%. This suggests that dialysis treatment may be associated with a higher risk of foot complications, consistent with the international literature.
The high prevalence of diabetic neuropathy and peripheral vascular disease observed in our sample (87% and 81.5%, respectively) highlights the impact of these complications on foot health in patients with DM and advanced CKD. Diabetic neuropathy, which affects a large proportion of patients with DM, is widely documented as a critical factor in DF development, as it reduces protective sensation in the feet, allowing minor injuries to progress undetected.11 In our study, patients on PD showed a slightly higher prevalence of neuropathy (89.5%) compared to those on HD, a finding consistent with that reported by Ndip et al.,5 who observed a relationship between dialysis treatment and increased incidence of neuropathy due to renal therapy-related factors and prolonged dialysis duration.
Peripheral vascular disease, present in 81.5% of our patients, is another condition that increases the likelihood of ulceration and severe foot complications. Patients on HD showed a higher prevalence of PVD (94.7%) compared to those on PD (74.3%). This finding could be attributed to hemodynamic changes and vascular stress associated with HD, which may exacerbate atherosclerosis and reduce blood flow to the extremities.4 Previous studies such as that by Jones et al.11 have suggested that HD, by inducing changes in blood pressure and peripheral vascular flow, may have an adverse effect on circulation in the limbs, worsening the risk of ulceration.
One of the notable aspects of our study is the high frequency of pre-ulcerative lesions, found in 61.1% of patients. These lesions, which include deformities and other pressure areas, are important predictors of active ulcer development. Early detection and management of these lesions are essential to prevent progression to ulcers and, eventually, amputations. Game et al.6 emphasize that pre-ulcerative lesions can be early indicators of risk and suggest that patients on RRT require constant monitoring to detect and manage these warning signs. In our study, the high frequency of pre-ulcerative lesions reinforces the need for a preventive approach in DF management, particularly within a multidisciplinary care setting.
Regarding history of amputations, 31.4% of patients in our sample had undergone at least one prior amputation, in line with studies documenting a correlation between dialysis duration and amputation risk in patients with DM.12 In the study by Margolis et al.,13 it was observed that diabetic patients on dialysis are at increased risk of amputation due to the combination of neuropathy, ischemia, and poor wound healing capacity – conditions exacerbated by RRT. The absence of significant differences in amputation rates between HD and PD in our sample may be explained by shared factors, such as prolonged DM duration and progression of both microvascular and macrovascular complications.
In line with our findings on the high prevalence of lesions and amputations in patients with DF under RRT, the recent study by Sánchez Correa et al.14 reported a high rate of reamputation in patients with minor foot amputations. These data reinforce the severity of the clinical course of DF and the need to implement early preventive and follow-up strategies, particularly in high-risk populations such as the one evaluated in our study.
Additional risk factors such as smoking and obesity were significantly more prevalent in the PD group compared to HD. These factors are not only independent contributors to DF development but also play a role in disease progression. Ndip et al.3 note that smoking worsens microcirculation and increases the risk of infection, while obesity contributes to excessive pressure on the foot and promotes the development of structural deformities. In our study, the prevalence of smoking and obesity in PD patients suggests that this group may benefit from specific lifestyle interventions to reduce the risk of DF complications.
This study provides relevant insight into the prevalence and risk factors of diabetic foot in patients with diabetes mellitus undergoing renal replacement therapy at a tertiary care hospital in Argentina. A key strength of this research is its focus on a high-risk population that has been underrepresented in national studies. This helps fill a gap in the literature regarding DF management in patients with advanced CKD on RRT. Moreover, the cross-sectional design allowed for the identification and characterization of specific factors associated with DF development in a real-world clinical setting, providing crucial data for the formulation of preventive interventions applicable to nephrology centers. The inclusion of a detailed assessment of key comorbidities, such as diabetic neuropathy and peripheral vascular disease, using standardized diagnostic methods, strengthens the consistency and accuracy of the findings.
Nonetheless, the study has certain inherent limitations. As a cross-sectional study, it is not possible to establish causal relationships between the identified risk factors and the development of DF complications, which limits the ability to interpret the temporal sequence of events. Additionally, the sample size, while adequate for descriptive analysis, may not be representative of larger populations or different healthcare settings, restricting the generalizability of the results. The absence of longitudinal data on the progression of foot lesions and the long-term effects of risk factors in patients with different RRT modalities also represents a limitation.
Future studies with a longitudinal design could provide a deeper perspective on the progression of DF in this population and assess the effectiveness of specific preventive interventions over time.
ConclusionThis study demonstrates the high prevalence of DF in patients with DM undergoing RRT at a tertiary care hospital in Argentina. Our findings, consistent with previous studies, show that patients on both hemodialysis and peritoneal dialysis are at high risk of foot complications, supporting the need for early prevention and management strategies. The incorporation of health programs focused on self-care, education on healthy lifestyle habits, and multidisciplinary follow-up could significantly reduce the incidence of ulcerations and amputations in this high-risk population.
Level of evidenceLevel of evidence III.
Ethical approvalThe Ethics Committee for Research Protocols of the Italian Hospital of Buenos Aires IRB No.: 10408.
FundingThis research has not received any specific support from public sector agencies, the commercial sector, or non-profit organizations.
Conflict of interestThe authors declare no conflicts of interest related to this work.