Alveolar hemorrhage syndrome can be secondary to multiple autoimmune disorders. The objective is to describe three diffuse alveolar hemorrhage (DAH) cases secondary to anti-synthetase syndrome (ASSD).
Presentation of the caseThree cases of ADH secondary to ASSD are described: one positive to anti-PL7, another positive to anti-PL12, and the last patient with double positivity to anti-Jo1 and anti-OJ. The patients presented improvement after receiving immunosuppressive treatment.
DiscussionThe evolution with therapeutic response and resolution of DAH supports the conclusion that ASSD is a potentially treatable cause of DAH and should be considered within the differential diagnosis in diagnosing DAH.
ConclusionThe described cases contribute to the knowledge of DAH, where ASSD should be considered in diagnosing DAH.
El síndrome de hemorragia alveolar puede ser secundario a múltiples padecimientos autoinmunes. El objetivo de este trabajo es describir 3 casos de hemorragia alveolar difusa (HAD) secundarios al síndrome antisintetasa (SAS).
Presentación del casoSe describen 3 casos de HAD secundaria al SAS: uno positivo a anti-PL7, otro positivo a anti-PL12, y el último paciente con doble positividad a anti-Jo1 y anti-OJ. Los pacientes presentaron mejoría después de recibir tratamiento inmunosupresor.
DiscusiónLa evolución con respuesta terapéutica y resolución de la HAD apoya la conclusión de que el SAS es una causa de la HAD potencialmente tratable, y que debe ser considerado dentro del diagnóstico diferencial en la evaluación diagnóstica de la HAD.
ConclusiónLos casos descritos contribuyen al conocimiento de la HAD, donde el SAS debe ser considerado una posible causa de la HAD.
DAH is a severe medical condition secondary to pulmonary microcirculatory injury that can be observed as a manifestation of autoimmune diseases, such as systemic lupus erythematosus or ANCA-associated vasculitis.1 Three histopathological patterns are associated with DAH: pulmonary capillaritis (the most common), soft pulmonary hemorrhage, and diffuse alveolar damage. The clinical manifestations of DAH include hemoptysis as the cardinal symptom, anemia, dyspnea, diffuse radiographic pulmonary infiltrates, and respiratory failure.1 The ASSD has been classified within the inflammatory myopathies. Nevertheless, the leading cause of morbidity and mortality in the ASSD is interstitial lung disease (ILD), with nonspecific interstitial pneumonia (NSIP) with or without organizing pneumonia (OP) being the main pattern of lung damage in this entity.2 There is scarce information about whether ASSD can present as DAH.3 This manuscript describes three cases of DAH secondary to ASSD and their evolution after treatment.
Patients and methodsPatients were evaluated at the Instituto Nacional de Enfermedades Respiratorias (INER) from 2014 to 2021. All patients evaluated at INER with suspected DAH undergo diagnostic bronchoscopy with bronchioalveolar lavage (BAL) and sampling for cultures. In addition, rheumatologists assess them clinically for the diagnosis of autoimmune diseases such as ANCA-associated vasculitis, systemic lupus erythematosus, and antiphospholipid syndrome. Clinical symptoms and signs related to the ASSD are evaluated as part of that evaluation. Moreover, the following anti-synthetase antibodies are measured: anti-Jo1, anti-PL7, anti-PL12, anti-EJ, and anti-OJ (EUROLINE myositis profile 3, positive result: unit density (UD)≥15).
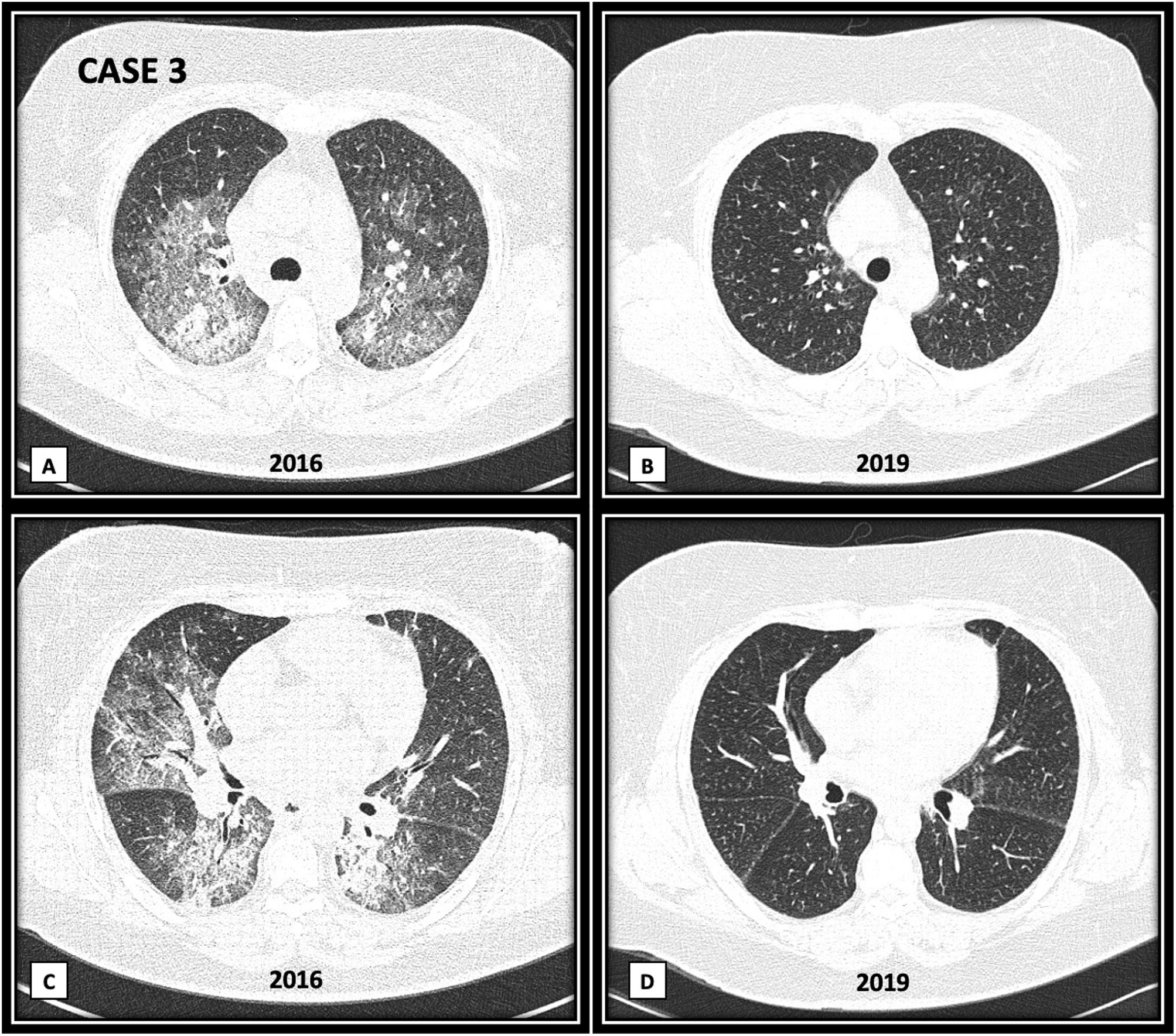
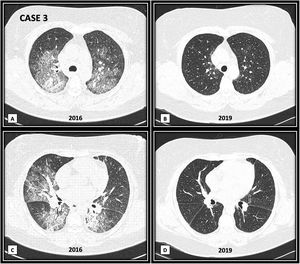
ResultsWe report three subjects with DAH as one of the first manifestations of ASSD. One patient was positive for anti-PL7, one positive for anti-PL12, and one with an overlap of two antibodies: anti-Jo1 and anti-OJ. In all cases, the diagnosis of DAH was confirmed by bronchoscopy, and the search for infectious agents by culture was negative. All subjects were ANCA, anti-PR3, and anti-MPO negative. One patient overlapped with another autoimmune disease: rheumatoid arthritis (RA). The three patients presented a favorable clinical evolution with remission of symptoms after receiving immunosuppressive treatment. The clinical improvement was evident within one week after the initiation of therapy. Table 1 describes the clinical manifestations of the cases, laboratory results, cabinet studies, and treatment. Fig. 1 shows a high-resolution tomography (HRCT) image of case 3 during DAH and a three-year follow-up HRC.
Description of the cases of DAH secondary to ASSD.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Sex | Male | Female | Female |
| Age (years) | 40 | 39 | 45 |
| Clinical manifestations | |||
| Dyspnea | Present | Present | Present |
| Cough | Present | Present | Present |
| Hemoptysis | Absent | Absent | Present |
| Myopathy | Absent | Absent | Absent |
| Arthritis/arthralgias | Absent | Absent | Present |
| Raynaud's phenomenon | Absent | Absent | Absent |
| Mechanic's hands | Present | Absent | Present |
| Fever | Present | Absent | Present |
| Proximal muscle weakness | Absent | Absent | Absent |
| Telangiectasias | Present | Absent | Present |
| Gottron papule sign | Present | Absent | Absent |
| HRCT (pattern) | NSIP/OP | OP | OP |
| ANA (≥1:80) | Cytoplasmic ribosomal 1:1280 | NuMA 1:160 | Nucleolar+fine speckle 1:160 |
| Anti-synthetase antibodies | Anti-PL12+++(125 DU) | Anti-Jo1+(15 DU), anti-OJ+(22 DU) | Anti-PL7+(32 DU) |
| ANCA | Negative | Negative | Negative |
| Anti-PR3 (UR/mL) | <2 | <2 | <2 |
| Anti-MPO (UR/mL) | <2 | <2 | <2 |
| Other antibodies | Anti-Ro52+++(117 DU) | None | Anti-Ro52+, anti-CCP>200U/mL |
| Complementary laboratories | |||
| Hemoglobin (g/dL) | 16.1 | 7.8 | 7 |
| CPK | 125 | 88.5 | 41 |
| CRP | 0.71 | 70.1 | 0.78 |
| Respiratory function tests | |||
| FVC predicted% | Were not performed | 67 | 75 |
| DLco predicted% | 80 | 77 | |
| Hemorrhagic bronchial alveolar lavage | |||
| Macrophages | * | 6 | 92 |
| Lymphocytes | * | 30 | 8 |
| Neutrophils | * | 64 | 0 |
| Eosinophils | * | 0 | 0 |
| Erythrocytes | Abundant | Abundant | Abundant |
| Hemosiderin-loaded macrophages | Present | Present | Present |
| Bronchoscopic diagnosis | Alveolar hemorrhage | Alveolar hemorrhage | Alveolar hemorrhage |
| Other CTD | None | None | RA |
| Treatment | HCT, PDN, MTX, LEF, RTX | HCT, PDN, LEF, MTX | MTX, LEF, PDN |
Abbreviations: ASSD, anti-synthetase syndrome; DAH, diffuse alveolar hemorrhage; RA, rheumatoid arthritis; HRCT, high-resolution tomography; DU, density unit; NSIP, non specific interstitial pneumonia; OP, organized pneumonia; CTD, connective tissue diseases; FVC, forced vital capacity; DLco, diffusing capacity for carbon monoxide; MTX, methotrexate; PDN, prednisone; RTX, rituximab; LEF, leflunomide; HCT, hydrocortisone.
(A and C) Bilateral patchy areas of ground glass, with crazy paving pattern. In basal lung areas (C image) there are areas of ground glass and consolidation with air bronchogram. (B and D) Three years follow-up images, showing complete disappearance of ground glass, crazy paving and consolidation areas.
We describe three cases of DAH in patients with ASSD and the resolution of DAH with clinical improvement after receiving treatment with high-dose corticosteroids in combination with methotrexate, leflunomide, or rituximab. The three cases were confirmed by bronchoscopy with an hemorrhagic BAL. Importantly, infectious diseases were ruled out. The presentation of these three cases may be helpful to clinicians evaluating patients with DAH.
DAH is a clinical syndrome with different etiologies, and autoimmune diseases are the leading cause.4 These three cases emphasize that the ASSD should be considered a possible etiology of DAH. The correct treatment of a patient with DAH could only begin after the proper diagnosis is made. If there is no clinical suspicion that the ASSD is a cause of DAH, clinicians may not evaluate a patient with DAH for the diagnosis of ASSD.
It is interesting to mention that two of the cases were also positive for Ro52 autoantibodies. This autoantibody has been associated with ILD severity in the ASSD.5,6 Another patient was positive for anti-citrullinated citric peptide antibodies (ACPA) positivity. It has been estimated that about 24% of ASSD are positive for ACPA antibodies.7 Another piece of relevant information is the heterogeneity of the anti-synthetase autoantibodies of the described cases. Although Jo1 is the most prevalent antibody in ASSD, the presented cases were positive for PL12, OJ, and PL7 autoantibodies. So, these anti-synthetase antibodies must be evaluated in the search for a specific diagnosis of DAH.
Our center has experience in the management of ASSD patients.8,9 Most of our patients receive a combination of high doses of corticosteroids (prednisone equivalent dose 50–75mg/day), methotrexate, and leflunomide.9 With this treatment, about 67% of the patients have lung improvement, as indeed was the case of these patients. The corticosteroid that we used was hydrocortisone 300mg/day, IV equivalent to 75mg of prednisone.
Autoimmune conditions such as ANCA-associated vasculitis or SLE are the principal causes of DAH secondary to pulmonary capillaritis.1,4 Although our cases do not have the pathological demonstration of pulmonary capillaritis, the response after treatment with corticosteroids and immunosuppression suggests that this mechanism may be responsible for the DAH. A limitation, besides the retrospective nature of the manuscript, is that our cases lack lung biopsy to confirm pulmonary capillaritis as the cause of DAH.
ConclusionIn conclusion, the described cases contribute to the knowledge of DAH syndrome, with the message that ASSD should be considered a possible cause of DAH. Patients with DAH should be evaluated for anti-synthetase antibodies, including anti-Jo1, anti-PL7, anti-PL12 and anti-OJ.
Ethical considerationsThe authors declare that this article does not contain personal information that allows the identification of the patients.
FundingThis research and the preparation of the article did not receive economic financing from any specific institution.
Conflict of interestThe authors declare no conflict of interest.