One-third of the world's population has latent tuberculosis. Because it can be reactivated by immunosuppressive treatments, screening is suggested. In Colombia, the tuberculin test with the booster is recommended in this population because of the risk of false negative results and anergy caused by treatments. Currently, the number of patients detected with this second test is unknown, so the primary objective is to determine the percentage of additional positive detections.
Materials and methodsFrom 2017 to 2022, a cross-sectional, analytical study was conducted on a group of patients from a rheumatology service who had a tuberculin test and a booster within a year to check for latent tuberculosis. Over the course of a year and three weeks, we looked at the number of patients whose booster tests showed new findings. A study was conducted to see if there were any differences in treatment based on the first tuberculin result.
ResultsA tuberculin test was performed on 674 subjects, of whom 496 were immunosuppressed. From the 382 whose test was negative, 191 and 74 booster tests were performed within a year and three weeks; 8 (4.1%) and 3 (3.6%) were positive, respectively. When evaluating the differences in treatments between the groups with a positive and negative first tuberculin test, significant differences were found. Use of prednisolone at 5mg or more per day (p=.02) and three immunosuppressants (p=.005) was more frequent in negative tuberculin tests.
ConclusionsIt can be said that the booster test raises the number of people with latent tuberculosis by 4%. More use of prednisolone at 5mg/day or three immunosuppressants in the group with the negative tuberculin test was found to be statistically significant.
Una tercera parte de la población tiene tuberculosis latente. Debido a que puede haber una reactivación por el uso de tratamientos inmunosupresores, se recomienda su cribado. En Colombia, la prueba de tuberculina con refuerzo es recomendada en esta población por el riesgo de falsos negativos relacionados con la anergia que causa el tratamiento. En la actualidad el número de pacientes detectados por esta segunda prueba es desconocido, por lo cual el objetivo es determinar el porcentaje de detecciones adicionales.
Materiales y métodosSe llevó a cabo un estudio analítico de corte transversal en un grupo de pacientes de un servicio de reumatología, en quienes se hizo prueba de tuberculina y refuerzo para estudio de posible tuberculosis latente en un periodo entre el 2017 y el 2022. Se revisó el número de detecciones nuevas por el refuerzo de tuberculina tomado en un periodo de un año y 3 meses. Se estudiaron las posibles diferencias entre los grupos con prueba inicial de tuberculina positiva o negativa.
ResultadosLa prueba de tuberculina se realizó en 674 pacientes, de los cuales 496 tenían terapia inmunosupresora, en tanto que 382 tenían prueba negativa. Se hizo una segunda prueba en solo 191 pacientes dentro del año siguiente a la primera prueba, de los cuales a 74 se hizo dicha prueba en las 3 semanas siguientes; 8 (4,1%) y 3 (3,6%) de estas pruebas fueron positivas, respectivamente. Al evaluar las diferencias en los tratamientos entre los grupos de primera prueba de tuberculina positiva o negativa se encontraron diferencias como un mayor uso de prednisolona a dosis de 5mg (p=0,02) o más al día y el uso de 3 o más inmunosupresores (p=0,005), siendo más frecuente el grupo con la prueba negativa.
ConclusiónSe puede decir que el refuerzo de tuberculina aumenta la detección en un 4%. Existe una relación entre el mayor uso de prednisolona y 3 o más inmunosupresores con el resultado de la primera prueba negativa.
Tuberculosis is a high-incidence infection in Colombia, with a rate of 22.6 cases per 100,000 people by 2020.1
The disease can manifest in different ways. One of these is the latent state, in which there are no signs that the disease is active. Patients with compromised function of the immune system, especially cellular immune response, have a higher risk of reactivation of the disease by 10–20%, with an increase in morbidity and mortality.2
When immunosuppressing drugs are used to treat rheumatological diseases, screening for latent tuberculosis with the gamma interferon-realized test or tuberculin is recommended.
Each of the strategies has advantages and disadvantages. In Colombia, according to the national guidelines for the diagnosis and treatment of tuberculosis in 2020, the use of the tuberculin test is recommended for the immunocompromised population, which will receive anti-TNF (tumor necrosis factor) biological therapy or prednisolone at a dose greater than or equal to 15mg/day for more than three months. It also recommends performing a booster if the first result is negative if immunosuppressive treatments have been used recently, concerning the anergy phenomenon, understood as the decreased response of CD4+ T cells to the exposure of antigens with a negative test, even if the immune system has been exposed to mycobacteria.3
The “booster” phenomenon has been defined by the CDC (Centers for Disease Control and Prevention) as the second positive test within a year after an initial negative reaction in previously infected patients because it “triggered the memory” of the immune system. They also suggest a two-step strategy for the second tuberculin skin test, which should be done 1–3 weeks after the first test. This is to make it less likely that the second test will be misread as a recent infection, especially in groups that will be tested often.3
In Mexico, a Latin American country with a disease burden similar to Colombia's, a group of immunocompromised patients who took the booster test found that the number of people with latent tuberculosis had increased by 15%. In Colombia, the additional cases that can be provided by the evidence of a booster test in this population are unknown, since we do not have data in this regard; It is necessary to assess the usefulness of the second test since its performance can delay the start of treatments and increase costs; in addition, it would be taken into account for Colombian guides in the area of rheumatology.
Because of this, it has been suggested to do an analytical observational study to find out how many more patients can be found by a booster test.
MethodsA cross-sectional and analytical study was conducted. We looked into the patient databases of a rheumatology service. We included patients who were 18 years of age or older, immunosuppressed by treatment for rheumatological conditions, and who had two tuberculin tests within a year.
Patients who had more than a year between their two tuberculin tests, were thought to have pulmonary or extra-pulmonary active tuberculosis, had a history of active or latent tuberculosis that had been treated partially or completely, had been infected by non-tuberculous mycobacteria, or were on immunosuppressive treatment for a disease other than rheumatoid arthritis were not included in the study.
Immunosuppressive therapy was defined as the use of at least one of the following drugs: methotrexate 15mg or more per week for 4 weeks, leflunomide 100mg per week for 4 weeks; sulfasalazine 1.5 gr per day or more for 4 weeks; prednisolone 7.5mg or more per day for 4 weeks, azathioprine 50mg or more per day for 4 weeks; mycophenolate mofetil 1g or more per day for 4 weeks; cyclophosphamide 500mg per intravenous pulse one or more times; biological therapy more than one dose applied. We did not include patients with small synthetic molecules or Janus kinase inhibitors because there were not any patients using this medication in the database. In Colombia, everyone should get the BCG vaccine at one-year old, so we expected not to have anyone with less than 15 years after vaccination, which was the reason to not include this criteria as an exclusion.
It was considered the first tuberculin test or booster to be negative between 0 and 4mm. The main objective was to determine the percentage of patients with latent tuberculosis detected by the use of the second tuberculin test if the second test was carried out within a year and 3 weeks. The secondary objectives were to describe the population of immunosuppressed patients according to the results of the first test, positive or negative, and find out if there is any difference in treatments between groups.
Based on previous studies that showed that using tuberculin reinforcement increased the number of positives by an average of 15%,3 the sample size was calculated assuming a much lower value, with a test positivity of 4% as an alternative hypothesis. The minimum number of patients required for an alpha error of 0.025 and a power of 80% is 189. The statistical analysis was carried out with the statistical program Stata 17, fed by the electronic database collection formats of the REDCap platform. In univariate analysis, the general description of subjects was performed according to the nature of the variables. The relative and absolute frequencies for qualitative variables were described; the respective measures of central tendency and dispersion for quantitative variables were also described; for variables with normal distribution, means with standard deviation and medians with interquartile ranges for those with no normal distribution were described. For the primary objective, the number of patients with a second positive tuberculin test and, therefore, a diagnosis of latent tuberculosis was described. For the secondary objectives, a bivariate analysis was performed by logistic regression or chi-square test according to the nature of the variables, and an adjusted regression was performed when some kind of difference was found. There were not any patients with HIV infection or active cancer, so these variables were not included in the analysis. A value of p less than 0.05 was considered statistically significant. The current study has received approval from an ethics committee and complies with the requirements set forth by the Colombian Ministry of Health.
ResultsFrom February 2017 to February 2022, a tuberculin test was done on a total of 674 patients with autoimmune diseases. Of these, 557 (82.6%) were women. Of the 496 patients who met the previous definition of immunosuppressed, 114 (22.9%) had a positive tuberculin test. Of the 178 non-immunosuppressed patients, 31 patients were found with a positive tuberculin test (17.4%). 382 immunosuppressed patients had a negative tuberculin test; among these, 191 patients received a tuberculin booster within a year and 74 within 4 weeks after, with 8 (4.1%) and 3 (3.6%) positive patients.
Patients who took the second tuberculin test were diagnosed with rheumatoid arthritis in 138 cases (72%), spondyloarthropathy in 19 cases (10%), psoriatic arthritis in 15 cases (8%), systemic erythematous lupus in 7 cases (3.5%), and other conditions in 12 cases.
In respect to treatments at immunosuppressive doses, 102 (53.6%) used methotrexate, 106 (55.5%) used leflunomide, 20 (10.5%) used sulfasalazine, 75 (39.2%) used prednisolone, 18 (9.4%) used biologic therapy, 6 (3%) used azathioprine, 1 (0.5%) used mycophenolate mofetil, and 3 (1%) used cyclophosphamide. The time of the booster test was between weeks 1 and 3 in 43.5%, weeks 3–12 in 13.5%, weeks 13–24 in 23%, and more than 24 in 20%.
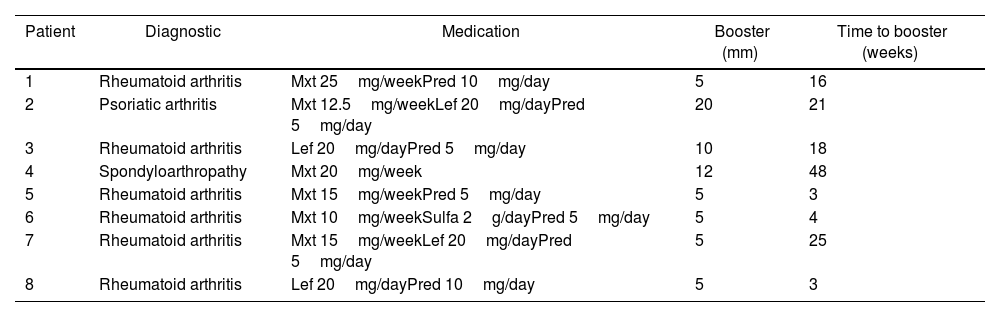
Table 1 summarizes the characteristics of patients with a positive booster test.
Description of immunosuppressed patients with a positive booster test.
| Patient | Diagnostic | Medication | Booster (mm) | Time to booster (weeks) |
|---|---|---|---|---|
| 1 | Rheumatoid arthritis | Mxt 25mg/weekPred 10mg/day | 5 | 16 |
| 2 | Psoriatic arthritis | Mxt 12.5mg/weekLef 20mg/dayPred 5mg/day | 20 | 21 |
| 3 | Rheumatoid arthritis | Lef 20mg/dayPred 5mg/day | 10 | 18 |
| 4 | Spondyloarthropathy | Mxt 20mg/week | 12 | 48 |
| 5 | Rheumatoid arthritis | Mxt 15mg/weekPred 5mg/day | 5 | 3 |
| 6 | Rheumatoid arthritis | Mxt 10mg/weekSulfa 2g/dayPred 5mg/day | 5 | 4 |
| 7 | Rheumatoid arthritis | Mxt 15mg/weekLef 20mg/dayPred 5mg/day | 5 | 25 |
| 8 | Rheumatoid arthritis | Lef 20mg/dayPred 10mg/day | 5 | 3 |
Mxt: methotrexate; Lef: leflunomide; Pred: prednisolone; Sulfa: sulfasalazine.
Of the 8 patients with positive Booster tests, 6 had a diagnosis of rheumatoid arthritis, 1 had psoriatic arthritis, and 1 had ankylosing spondylitis. Prednisolone was used in 87.5% (7 patients) at 5mg/day (2–10mg/day), methotrexate in 75% (6 patients), leflunomide in 50% (4 patients), and sulfasalazine in 12.5% at 2g/day (1 patient). The results of the booster test were 5mm in 5 patients, performed between 3 and 25 weeks, 1 patient with 10mm, performed at 18 weeks, 1 patient with 12mm, performed at 48 weeks, and 1 patient with 20mm performed at 21 weeks.
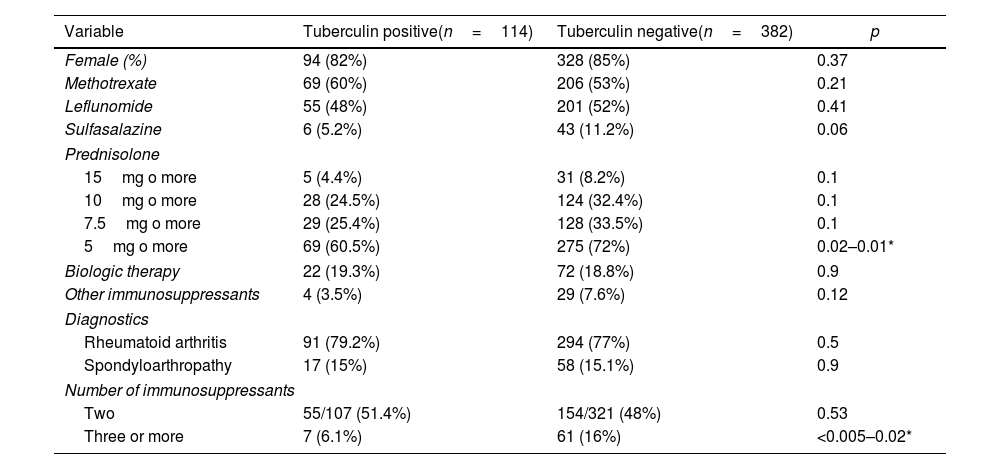
Table 2 shows the differences in treatments and diagnoses among immunosuppressed patients according to their results in the initial tuberculin test. Different doses of prednisolone were established due to disagreement over the immunosuppressive dose as a risk for reactivation of latent tuberculosis. In other immunosuppressors, azathioprine, mycophenolate mofetil, and cyclophosphamide were grouped due to their low frequency of use; likewise, biological therapies were considered a unique variable.
Differences between groups with positive and negative tuberculin tests in immunosuppressed patients.
| Variable | Tuberculin positive(n=114) | Tuberculin negative(n=382) | p |
|---|---|---|---|
| Female (%) | 94 (82%) | 328 (85%) | 0.37 |
| Methotrexate | 69 (60%) | 206 (53%) | 0.21 |
| Leflunomide | 55 (48%) | 201 (52%) | 0.41 |
| Sulfasalazine | 6 (5.2%) | 43 (11.2%) | 0.06 |
| Prednisolone | |||
| 15mg o more | 5 (4.4%) | 31 (8.2%) | 0.1 |
| 10mg o more | 28 (24.5%) | 124 (32.4%) | 0.1 |
| 7.5mg o more | 29 (25.4%) | 128 (33.5%) | 0.1 |
| 5mg o more | 69 (60.5%) | 275 (72%) | 0.02–0.01* |
| Biologic therapy | 22 (19.3%) | 72 (18.8%) | 0.9 |
| Other immunosuppressants | 4 (3.5%) | 29 (7.6%) | 0.12 |
| Diagnostics | |||
| Rheumatoid arthritis | 91 (79.2%) | 294 (77%) | 0.5 |
| Spondyloarthropathy | 17 (15%) | 58 (15.1%) | 0.9 |
| Number of immunosuppressants | |||
| Two | 55/107 (51.4%) | 154/321 (48%) | 0.53 |
| Three or more | 7 (6.1%) | 61 (16%) | <0.005–0.02* |
Methotrexate: ≥15mg/week; leflunomide: ≥100mg/week; sulfasalazine: ≥1g/day.
Statistically significant differences were found in the use of prednisolone at doses equal to or greater than 5mg/day (p=0.01) and the use of three or more immunosuppressants (p=0.02), both more frequently used in the negative tuberculin test group. Even when other treatment factors, like methotrexate, leflunomide, sulfasalazine, prednisolone, biologic therapy, azathioprine, mycophenolate mofetil, and cyclophosphamide, were taken into account, the differences between the groups remained.
DiscussionIn the 2020 Colombian guidelines for the diagnosis and management of tuberculosis, in its section on the diagnosis and treatment of latent tuberculosis, it is recommended the active search for latent tuberculosis in subjects in the condition of immunosuppression and the performance of a second test of a purified protein derivative for the evaluation of the booster effect by above the measurement of the release of interferon-gamma, preferably by the third week of the first test.4
In our study, we collected a total of 674 patients with immunosuppression because of the autoimmune disease treatment, finding a prevalence of latent tuberculosis of 21.5%, a higher value than found in the studies of Santos-Moreno et al., Bautista-Molano et al., and Mora et al. of 8–18% in patients with autoimmune disease, specifically rheumatoid arthritis (RA), without the use of biological therapy, and a similar prevalence found by Galindo et al. (21.5%) in a population with oncological pathology prior to initiation of chemotherapy.5–8 It is important to highlight the low prevalence found in the study of Santos-Moreno, which may be associated with the use of biological therapy in 77% of the population, raising a possible phenomenon of secondary anergia.
Literature about tuberculin reinforcing or boosting is scarce in autoimmune and inflammatory diseases, and even more so in areas with a high prevalence of tuberculosis. In Mexico, a country with a prevalence close to that of Colombia, Pérez-Barbosa et al. found, in 143 patients with rheumatoid arthritis, a positive tuberculin test in 39 patients (27.2%). In patients with negative results, 104 (72.7%) boosters were performed in 84 patients (58.7%), of which 9 (6.3%) presented positive tests, with a final latent tuberculosis prevalence of 33.5%, higher in the early RA group (<52 weeks) (OR 1.3, 95% CI (1.007–1.7), p=0.04). Their work concludes that performing boosters increases the detection of latent tuberculosis in this population by 15% (p=0.048).3
In our research, the booster was positive in 8 patients (4.1%), which increases the prevalence of latent tuberculosis to 22.7% in the population with rheumatological diseases and 24.5% in the group of patients with the use of immunosuppressive drugs.
We also analyzed the number of positive booster tests within 3 weeks because it is recommended to reduce misinterpretation; however, a very close number of 3.6% was found for a one-year retest, which can reinforce the value of having the second test even longer than 3 weeks, taking into account a variety of difficulties to having the test in this period of time in our countries, with almost half of the patients having the test later than this time.
About 5–10% of people with latent tuberculosis will develop active tuberculosis at some point in their lives, with the risk being highest in the first 5 years after the initial infection.9 This increases between 10 and 20% with factors that modify the functioning of the immune system, like recent conversion of PPD, under 35 years old who are receiving infliximab therapy, an induration of 10mm, a human immunodeficiency virus infection, or a history of tuberculosis infection with cure criteria.2 We, therefore, consider that the 4% increase in the diagnosis of latent tuberculosis has a significant value in this group of patients because it allows the identification of a greater proportion of patients with a high probability of reactivation due to their immunosuppressive therapy.
Only half of the patients in the group with low immune function followed the recommendation to do a second tuberculin test. This is probably because there is no standard recommendation for the second test in international and national guidelines for screening for latent tuberculosis in rheumatological diseases.
We also found a wide difference between the times of execution of the booster, both factors were perhaps associated with unknowingness of the importance of the second test, administrative limitations by health insurers, and the availability of testing laboratories.
Anergia is the decreased response of CD4+ T cells to antigens in the tuberculin test in immunocompromised patients. Negative results don’t rule out contact with mycobacteria, even though the test is negative. Vallejo et al. found in a group of 100 patients with RA that anergy for PPD was present in 9%; all patients used corticosteroids, 44% methotrexate, and 33% biological therapy, which was a risk factor for anergia (OR 1.096, 95% CI 1.016–1.18, p<0.05) like the use of prednisolone at doses of at least 5mg/day (OR 1.044, 95% CI 1.008–1080, p<0.05).10
In our study, we found that greater use of prednisolone at a dose of 5mg/day (p=0.01) and three or more immunosuppressants at a time (p=0.02) may be related to the phenomenon of anergia, which would reinforce the need for standardization of the performance of the booster test.
Within the limitations of our study, we consider that the sample size could not allow us to assess statistically significant differences between other immunosuppressive pharmacological groups other than corticosteroids; In addition, it is considered that factors such as age and disease activity, which may have a relation to the state of anergia, may be the subject of further studies in this area.
We can conclude that performing the booster test in a population of immunosuppressed patients with an autoimmune disease increases the prevalence of latent tuberculosis by 4%, which is a relevant result considering the higher risk of reactivation of the disease. In addition, the use of prednisolone or a combination of immunosuppressive agents may be related to the state of anergy.
Ethical considerationsThe present study meets the requirements stipulated by the Ministry of Health of Colombia and is approved by the Unisanitas Ethics Committee- Bogotá / Colombia CEIFUS 2560-22 protocol 096-22 UNV.
Statements and declarationsThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.
This study was not funded.
The authors declare that no material has been produced partially or entirely with the assistance of artificial intelligence.
The authors' contributions were in different stages of the study. LC contributed to data collection and statistical analysis. MT and MC contributed to data collection and article writing.