Anti-tumor necrosis factor-alpha (TNF-α) treatments have been available for over two decades to treat inflammatory arthropathies (IA). Most of these disorders are common among women of reproductive age, which emphasizes the need to evaluate their safety in pregnancy.
ObjectiveThis study aims to scrutinize neonatal and pregnancy outcomes in pregnant IA patients treated with adalimumab.
Materials and methodsThe current cross-sectional work was conducted by reviewing the medical files of pregnant IA patients (n=30) receiving adalimumab referred to Golestan Hospital in Ahvaz (Iran) from 2014 to 2017, followed by extracting demographic profiles as well as neonatal and pregnancy outcomes.
ResultsNoteworthy among the findings were PsA (n=13), RA (n=5), IBD (n=4), AS (n=3), uveitis (n=2), Behcet's disease (n=2), and panuveitis (n=1). The mean age of subjects, duration of illness, and duration of treatment were estimated at 29.53±5.88, 2.85±1.15, and 1.96±.90 years, respectively. No delivery outcome was found for 27 (90%) cases, and delivery outcomes observed in three (10%) patients were abortion (n=2) and preterm complications (n=1). No neonatal complication was found for 28 (93.3%) cases and neonatal IUGR outcome was reported in 2 (6.7%) cases. Cesarean section was a delivery method in 7 (23.3%) cases and natural method in 21 (70%) cases. There were no significant differences for the prevalence of cesarean section and neonatal outcomes based on the type of disease, but differences were observed for the outcome of delivery based on the type of disease.
ConclusionAccording to our findings, definitive conclusions on the safety of adalimumab during pregnancy were impossible and there is a need for further research with a larger sample size.
Los tratamientos antifactor de necrosis tumoral alfa (TNF-α) están disponibles desde hace más de dos décadas para el tratamiento de las artropatías inflamatorias (AI). La mayoría de estos trastornos son comunes entre las mujeres en edad reproductiva, lo que enfatiza la necesidad de evaluar su seguridad durante el embarazo.
ObjetivoEste estudio tiene como objetivo analizar los resultados neonatales y del embarazo en pacientes embarazadas con AI tratadas con adalimumab.
Materiales y métodosEl trabajo transversal actual se realizó mediante la revisión de los expedientes médicos de pacientes embarazadas con AI (n=30) que recibieron adalimumab y que fueron derivadas al Hospital Golestan en Ahvaz (Irán) entre los años 2014 y 2017, seguida de la extracción de perfiles demográficos y resultados neonatales y del embarazo.
ResultadosLos hallazgos destacaron PsA (n=13), AR (n=5), EII (n=4), AS (n=3), uveítis (n=2), enfermedad de Behçet (n=2) y panuveítis (n=1). La edad media de los sujetos, la duración de la enfermedad y la duración del tratamiento se estimaron en 29,53±5,88, 2,85±1,15 y 1,96±0,90 años, respectivamente. No se encontró ningún resultado del parto en 27 (90%) casos, en tanto que los resultados del parto observados en 3 (10%) pacientes fueron aborto (n=2) y complicaciones pretérmino (n=1). No se encontró ninguna complicación neonatal en 28 (93,3%) casos, y se informó el resultado de retraso del crecimiento intrauterino (RCIU) neonatal en 2 (6,7%) casos. La cesárea fue el método de parto en 7 (23,3%) casos, mientras que el método natural lo fue en 21 (70%) casos. No hubo diferencias significativas para la prevalencia de cesárea y los resultados neonatales según el tipo de enfermedad, pero sí para el resultado del parto según el tipo de enfermedad.
ConclusiónSegún nuestros hallazgos, las conclusiones definitivas sobre la seguridad de adalimumab durante el embarazo fueron imposibles, por lo cual es necesario realizar más investigaciones con un tamaño de muestra más grande.
Adalimumab (ADA) is a biologic medication extensively prescribed to treat immune-mediated inflammatory diseases (IMID) like psoriasis (PS), rheumatoid arthritis (RA) and inflammatory bowel disease (IBD). There is still debate about the effect of adalimumab on mother and baby during pregnancy, highlighting the need to be fully aware of the impacts of this therapy on neonatal and pregnancy outcomes. Hormonal status alters during pregnancy; for example, the level of estrogen is elevated that leads to stimulation of immunological responses mediated by T-helper cell (Th) 2, and suppression of Th1 cytokines. Therefore, pregnant patients with Th2-caused disorders, like ulcerative colitis and other diseases of the immune system, may suffer from flare-ups. Excessive activity and poor control of disease in pregnant women may influence the pregnancy outcome. Optimal and controlled treatment of immune system diseases is necessary during pregnancy. Although a pregnant patient can show improvement in the disease, continued anti-tumor necrosis factor-alpha (TNF-α) treatments can help prevent flare-ups accompanied with an elevated risk of low birth weight, preterm delivery and miscarriage. The TNF-α therapy is capable of regulating the placental hormones and modulating trophoblast invasion and proliferation, thus influencing the pregnancy outcome. The TNF-α dysregulation in pregnancy can cause some adverse effects such as spontaneous abortion and preterm birth.
Some of the conditions in the category of inflammatory arthropathies (IA) include ankylosing spondylitis (AS), psoriatic arthritis (PsA) and rheumatoid arthritis (RA). The difference in these diseases can be attributed to methods of treatment, clinical phenotype and mechanisms of pathogenesis. These diseases begin in the third to sixth decade of life. Hence, the majority of female IA patients are of childbearing age, with a worrying interaction between pregnancy outcome and disease.1,2 Most available information on the association between rheumatic inflammatory disease and pregnancy has been achieved from studies on RA patients, and there is limited pregnancy information for patients with other types of IA.2 According to findings, 70–80% of female RA patients exhibit improvement in the disease symptoms or declined activity during pregnancy, as well as 20% exhibit moderate or severe activity of illness during pregnancy probably requiring additional therapies.1 Although there have been many advances in treatment strategies, medical management has worrisome challenges for pregnant women suffering from inflammatory diseases due to potential fetal toxicity.3 Overall, the available protocols to manage pregnant RA patients recommend discontinuation of ritoximab, methotrexate and some other drugs before a planned pregnancy, while the administration of cyclosporine and antimalarial drugs during pregnancy may not be contraindicated.4 The monoclonal antibody of adalimumab can target the TNF-α, which needs active transport to cross the placenta because of its high molecular weight.5 Passage of this drug through the placenta can reportedly occur in the third month of pregnancy. Since adalimumab has just been introduced to the market, there is limited information on the safety profile of this drug during pregnancy.6 Little evidence has been documented in Asian countries about the safety of adalimumab treatment among pregnant women, and most published information is in the form of case reports or investigations with a small ample size. There is no report in Iran about the safety of this drug during pregnancy and no specific recommendation is available for the administration of this group of drugs to IA patients during pregnancy. Given the risks explained for IA patients and the need to identify safe treatments during their pregnancy, the present retrospective study aims to scrutinize the neonatal and pregnancy outcomes in pregnant IA patients treated with adalimumab.
Materials and methodsEthical considerationsThe research followed the tents of the Declaration of Helsinki. The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, also approved this study. The institutional ethical committee at Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, approved all study protocols (IR.AJUMS.HGOLESTAN.REC.1399.158). Accordingly, written informed consent taken from all participants before any intervention. This study was extracted from Internal Medicine Residency thesis of Navid Najarpour at this university (Thesis#U-99358).
Demographic profiles of study participantsThe current retrospective descriptive-analytical epidemiology research was carried out in 2020 on pregnant patients with IA (RA, PsA or AS) under treatment with adalimumab admitted to Golestan Hospital of Ahvaz (Iran) during 2016–2020. Inclusion criteria were ≤18 years of age, complete information of patient, and treatment with adalimumab for IA up to six months prior to pregnancy or during pregnancy minimally three months or 12 doses. Exclusion criteria were incomplete information or records of patient and history of underlying diseases in lung, heart, kidney or others.
Data collectionThe patients’ records were reviewed to extract contextual information (including gender, age, medical history, underlying conditions, smoking, body mass index (BMI) and medication) and pregnancy-related data (including time of delivery, gestational age, type of childbirth as cesarean or natural), indication for taking adalimumab (such as RA or lupus) and information on adalimumab therapy, maternal complications, and pregnancy and neonatal outcomes. The pregnancy outcomes were premature abortion, miscarriage, stillbirth, live birth (&#¿;37 weeks or ≥37 weeks), preterm labor (&#¿;37 weeks). The neonatal outcomes were minor and major birth defects and birth weight (&#¿;2500g or ≥2500g). Pregnancy-related maternal complications were gestational hypertension, arrhythmia, anemia, herpes simplex, upper respiratory tract viral infection (yes or no), hematoma, and severity of adverse effects (serious or non-serious) and their relationship to etanercept (related or not related or possibly related or unspecified).
Statistically data analysisThe collected data were statistically analyzed by SPSS version 22 software using descriptive tests of frequency, mean and standard deviation, as well as analytical tests of Chi-square test and independent t-test at the significance level of 0.05.
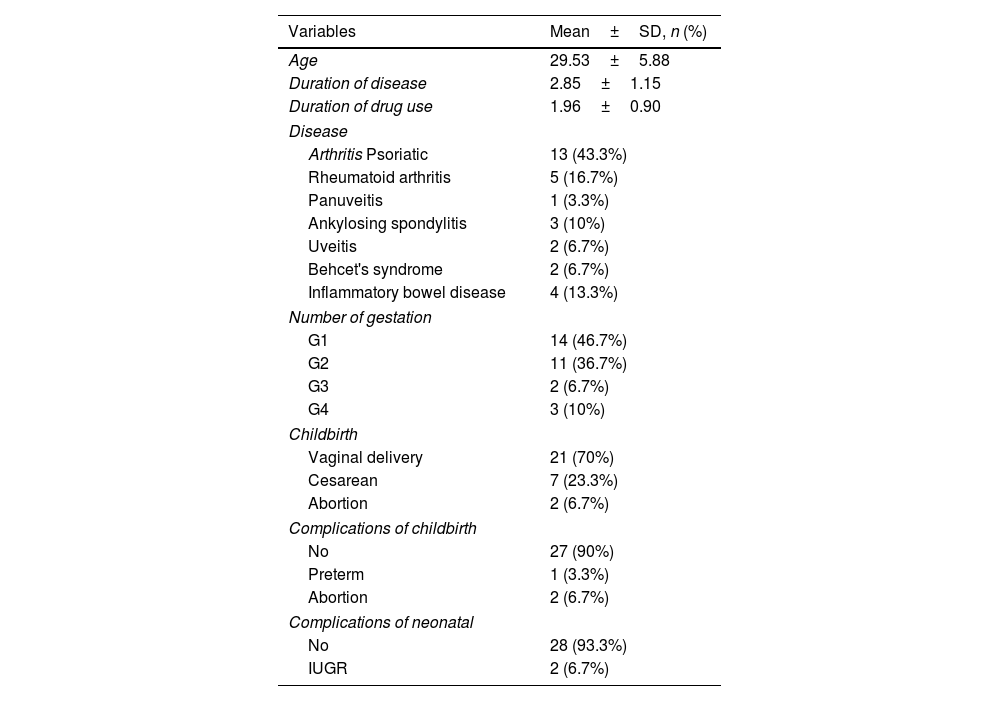
ResultsTable 1 represents the results of patient contextual information. The mean age of subjects, duration of illness and duration of drug treatment were estimated at 29.53±5.88, 2.85±1.15 and 1.96±0.90 years, respectively. Among a total of tested patients (n=30), the findings underlined PsA (n=13, 43.3%), RA (n=5, 16.7%), IBD (n=4, 13.3%), AS (n=3, 10%), uveitis (n=2, 6.7%), Behcet's disease (n=2, 6.7%) and panuveitis (n=1, 3.3%). Moreover, one pregnancy was observed in 14 (46.7%) cases, two pregnancies in 11 (36.7%) cases, three pregnancies in 2 (6.7%) cases and four pregnancies in 3 (10%) cases. Table 2 shows the findings regarding the distribution and frequency of delivery outcome types as well as neonatal outcome types. No delivery outcome was found for 27 (90%) cases, and delivery outcomes observed in three (10%) patients were abortion (n=2) and preterm complications (n=1). Cesarean section was a delivery method in 7 (23.3%) cases and natural method in 21 (70%) cases. No neonatal complication was found for 28 (93.3%) cases and neonatal IUGR outcome was reported in 2 (6.7%) cases.
Patient demographic profile.
| Variables | Mean±SD, n (%) |
|---|---|
| Age | 29.53±5.88 |
| Duration of disease | 2.85±1.15 |
| Duration of drug use | 1.96±0.90 |
| Disease | |
| Arthritis Psoriatic | 13 (43.3%) |
| Rheumatoid arthritis | 5 (16.7%) |
| Panuveitis | 1 (3.3%) |
| Ankylosing spondylitis | 3 (10%) |
| Uveitis | 2 (6.7%) |
| Behcet's syndrome | 2 (6.7%) |
| Inflammatory bowel disease | 4 (13.3%) |
| Number of gestation | |
| G1 | 14 (46.7%) |
| G2 | 11 (36.7%) |
| G3 | 2 (6.7%) |
| G4 | 3 (10%) |
| Childbirth | |
| Vaginal delivery | 21 (70%) |
| Cesarean | 7 (23.3%) |
| Abortion | 2 (6.7%) |
| Complications of childbirth | |
| No | 27 (90%) |
| Preterm | 1 (3.3%) |
| Abortion | 2 (6.7%) |
| Complications of neonatal | |
| No | 28 (93.3%) |
| IUGR | 2 (6.7%) |
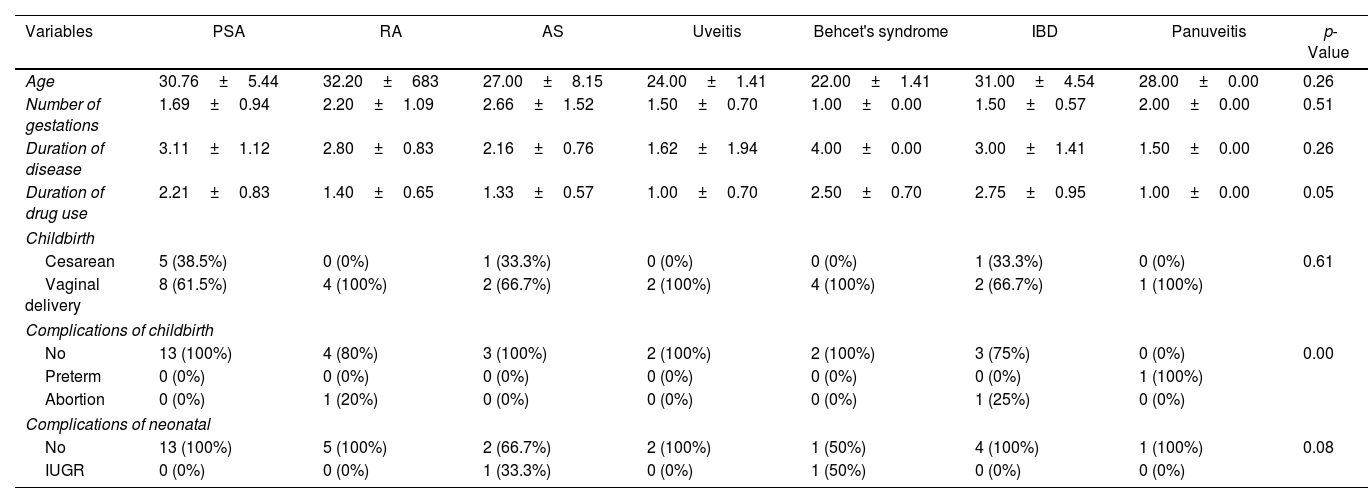
Results of patient characteristics by disease type.
| Variables | PSA | RA | AS | Uveitis | Behcet's syndrome | IBD | Panuveitis | p-Value |
|---|---|---|---|---|---|---|---|---|
| Age | 30.76±5.44 | 32.20±683 | 27.00±8.15 | 24.00±1.41 | 22.00±1.41 | 31.00±4.54 | 28.00±0.00 | 0.26 |
| Number of gestations | 1.69±0.94 | 2.20±1.09 | 2.66±1.52 | 1.50±0.70 | 1.00±0.00 | 1.50±0.57 | 2.00±0.00 | 0.51 |
| Duration of disease | 3.11±1.12 | 2.80±0.83 | 2.16±0.76 | 1.62±1.94 | 4.00±0.00 | 3.00±1.41 | 1.50±0.00 | 0.26 |
| Duration of drug use | 2.21±0.83 | 1.40±0.65 | 1.33±0.57 | 1.00±0.70 | 2.50±0.70 | 2.75±0.95 | 1.00±0.00 | 0.05 |
| Childbirth | ||||||||
| Cesarean | 5 (38.5%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 0.61 |
| Vaginal delivery | 8 (61.5%) | 4 (100%) | 2 (66.7%) | 2 (100%) | 4 (100%) | 2 (66.7%) | 1 (100%) | |
| Complications of childbirth | ||||||||
| No | 13 (100%) | 4 (80%) | 3 (100%) | 2 (100%) | 2 (100%) | 3 (75%) | 0 (0%) | 0.00 |
| Preterm | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | |
| Abortion | 0 (0%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | |
| Complications of neonatal | ||||||||
| No | 13 (100%) | 5 (100%) | 2 (66.7%) | 2 (100%) | 1 (50%) | 4 (100%) | 1 (100%) | 0.08 |
| IUGR | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 1 (50%) | 0 (0%) | 0 (0%) | |
Table 2 shows the findings for patient characteristics by disease type. No significant correlation was found between the mean age and the disease type (p=0.264). No significant correlation was found between the disease type and the mean number of pregnancies (p=0.515). No significant correlation was found between the mean disease duration and the disease type (p=0.265). No significant correlation was found between the mean duration of drug taking and the disease type (p=0.051). No significant correlation was found between the delivery type and the disease type (p=0.612). No significant correlation was found between the neonatal outcome and the disease type (p=0.081). A significant correlation was found between the delivery outcome and the disease type (p=0.000), such that the incidence of preterm was 100% in the patients with panuveitis, while the preterm delivery was observed in none of the other diseases. There was an abortion in 25% of IBD cases and in 20% of RA cases, but not in other diseases.
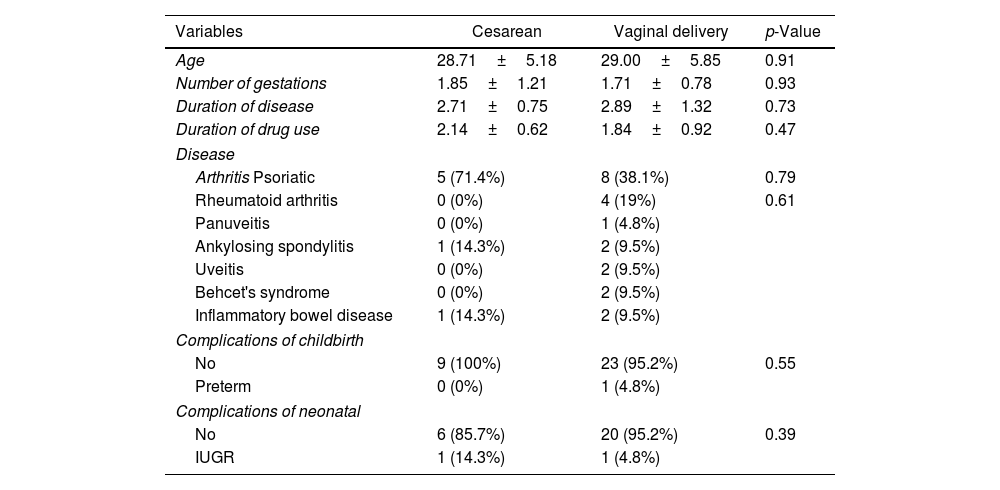
As seen in Table 3, the mean age of those with cesarean section and natural childbirth were 28.71±5.18 and 29.00±5.85 years, but not significant (p=0.910). The mean number of pregnancies in those with cesarean section (1.85±1.21) exhibited no significant difference than those with natural childbirth (1.71±0.78) (p=0.931). The mean illness duration in those with cesarean section (2.71±0.75) was not significantly different than those with natural childbirth (2.89±1.32) (p=0.739). The mean duration of drug taking in those with the cesarean section (2.14±0.62) exhibited no significant difference with those with natural childbirth (1.84±0.99) (p=0.472). No significant correlation was found between the neonatal outcome and the delivery type (p=0.397). No neonatal outcome was observed in 6 (85.7%) cases with cesarean section and the IUGR complication was seen in one (14.3%) case with cesarean section. No neonatal outcome was observed in 20 (95.2%) cases with natural childbirth and the IUGR complication was seen in one (4.8%) case with natural childbirth.
Results of patient characteristics by delivery type.
| Variables | Cesarean | Vaginal delivery | p-Value |
|---|---|---|---|
| Age | 28.71±5.18 | 29.00±5.85 | 0.91 |
| Number of gestations | 1.85±1.21 | 1.71±0.78 | 0.93 |
| Duration of disease | 2.71±0.75 | 2.89±1.32 | 0.73 |
| Duration of drug use | 2.14±0.62 | 1.84±0.92 | 0.47 |
| Disease | |||
| Arthritis Psoriatic | 5 (71.4%) | 8 (38.1%) | 0.79 |
| Rheumatoid arthritis | 0 (0%) | 4 (19%) | 0.61 |
| Panuveitis | 0 (0%) | 1 (4.8%) | |
| Ankylosing spondylitis | 1 (14.3%) | 2 (9.5%) | |
| Uveitis | 0 (0%) | 2 (9.5%) | |
| Behcet's syndrome | 0 (0%) | 2 (9.5%) | |
| Inflammatory bowel disease | 1 (14.3%) | 2 (9.5%) | |
| Complications of childbirth | |||
| No | 9 (100%) | 23 (95.2%) | 0.55 |
| Preterm | 0 (0%) | 1 (4.8%) | |
| Complications of neonatal | |||
| No | 6 (85.7%) | 20 (95.2%) | 0.39 |
| IUGR | 1 (14.3%) | 1 (4.8%) | |
Out of 30 patients, 27 (90%) had no childbirth complications but 3 patients (10%) experienced childbirth complications including preterm labor in 1 case and miscarriage in 2 cases. Also, 28 patients (93.3%) had no neonatal complication but 2 patients (6.7%) experienced neonatal complications, which was IUGR in both cases.
DiscussionWomen of reproductive age are often influenced by IA and autoimmune disorders like RA and IBD. Moreover, hormonal status alters during pregnancy; for example, the level of estrogen is elevated that leads to stimulation of immunological responses mediated by Th2, and suppression of Th1 cytokines. Therefore, pregnant patients with autoimmune diseases and RA may suffer from flare-ups. They may experience the disease development and progression.3 Greater activity of disease and negligible control of disease in pregnant women may influence the pregnancy outcome.7 The optimal and sufficient therapy of autoimmune and rheumatic disorders is necessary during pregnancy.8 The biological agents targeting TNF-α are predominantly prescribed to treat these diseases. However, there is incomplete information about their safety during pregnancy and additional research is needed to develop these data.9 The anti-TNF-α monoclonal antibody of adalimumab has been proposed to manage AS, RA and many other diseases.10 Little evidence has been documented in Asian societies regarding the safety of adalimumab treatment among pregnant women, and most published information is in the form of case reports or investigations with a small ample size and contradictory findings.11 Kawai et al. in 2018 examined pregnancy outcomes among adalimumab-treated patients suffering from ulcerative colitis, Crohn's disease, and RA (n=73), the results of which showed live births in 45 (84.9%) cases and abortion or stillbirth or induced abortion in 8 (15.1%) cases. Moreover, 3 (5.6%) infants had low birth weight. To conclude, the adalimumab safety was seen during pregnancy and no additional risk was there for pregnancy outcomes.3 Johnson et al. in 2009 compared the pregnancy outcome in pregnant RA women receiving adalimumab during the first pregnancy trimester with the pregnant RA women not receiving adalimumab and healthy pregnant women. The live births in the three groups were 83.3%, 90.9% and 85.5%, sequentially. Stillbirths and spontaneous abortions were 14.7%, 5.5% and 3.8% in the three groups, sequentially. No especial concern was reported regarding elevated risk of pregnancy complications following the administration of adalimumab for the RA treatment. They suggested further research with larger sample size because of little elevation in adverse outcomes mediated by adalimumab administration.12 In our work, out of 30 adalimumab-treated patients, 28 (93.4%) cases had live births, 2 (6.6%) cases had abortions and 2 (6.7%) cases reported IUGR, in fact less than findings of Kawai et al. that means the drug safety.
A study reported the pregnancy outcomes in patients with RA, IBD and other autoimmune diseases among patients receiving TNF-α inhibitors. In the patients receiving TNF-α inhibitors (4%) when comparing with those not receiving (88.3%), there were no differences in the spontaneous abortion (13.2% versus 9.6%), the preterm birth (16.5% versus 9.1%), and the low birth weight (14.8% versus 11.1%).13 A study on Japanese IBD patients, a significant elevation was seen in low birth weight (23.5% versus 24.1%), preterm births (5.9% versus 6.9%), congenital abnormalities (0% versus 6.5%) and mean birth weight (2844g versus 2920g) in patients with (n=24) and without anti-TNF treatment.14 The current work exhibited the frequency of IUGR complication (6.7), abortion (6.6%) and preterm (3.3%), which means less than systematic study but similar to the study in Japan possibly because of differences in demographic profile, sample size, anti-TNF drug type, time of exposure, duration of exposure, and so on.
A study reported that, out of 495 anti-TNF drug-exposed pregnant patients in comparison to 1532 non-exposed patients, there was an increase in major birth defects (5% versus 1.5%), low birth weight (12.8 versus 11.1) and preterm delivery (17.6% versus 9%) but not increase in miscarriage (5.9% versus 7.9%).15 In our work, there was natural delivery in 21 (70%) cases and cesarean section in 7 (23.3%) cases. Bröms et al. in 2020 reported that the risk of cesarean section (odds ratio=1.57) was higher in anti-TNF-treated women than that in non-exposed women. The cesarean section had greater prevalence in anti-TNF drug-exposed women (37.7%) than in non-exposed women (27.4%) and in control group (17.4%). The incidence rate of cesarean section was 30.9% in women exposed to etanercept, 47.1% in those exposed to infliximab and 40.1% in those exposed to adalimumab.16 The lower values calculated in our work than the study of Bröms et al. might be attributed to small sample size.
In our study, there was a significant correlation between the pregnancy outcome and the disease type, such that the incidence of preterm in patients with panuveitis was 100%, while preterm labor complication was seen in none of the other diseases. There was abortion in 20% of RA cases and in 25% of IBD cases, but these complications were not in other diseases. Moreover, the incidence of cesarean section and neonatal outcomes had no significant difference in the disease type. Nevertheless, it is difficult to reach definitive conclusions because of limited sample size in this study and each group.
Limitations of the studyOne of the limitations of this study was the small size of the sample because of the limited number of patients. Therefore, further studies with larger samples and with control groups are needed before definite conclusions can be drawn regarding the side effects of adalimumab. To do so, future studies also need to consider the relationship between neonatal and childbirth complications and the type of disease, type of TNF drug used, exposure duration and time (first, second, or third trimester), underlying diseases, etc.
ConclusionThe incidence rate of neonatal and cesarean section outcomes had no significant difference in the disease type, but the pregnancy outcome was different in terms of the disease type. However, we have reached conclusions regarding the safety of adalimumab during pregnancy and suggest further research with larger sample size.
Ethical considerationsIn addition, informed consent of the patients was obtained.
FundingThis project was funded by Ahvaz Jundishapur University of Medical Sciences.
Conflict of interestsThe authors declare that they have an absence of any conflict of interest for the preparation of this manuscript.
We would also like to show our gratitude to the Clinical Research Development Unit, Golestan Hospital, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran for sharing their pearls of wisdom with us during the course of this research.