Palinopsia, described as the persistence of visual images or their recurrence after the stimulus has disappeared, represents a rare but important neuro-ophthalmic complication. Until now, the pathophysiology and the main location of this visual mainteanance condition are not been clearly understood. This study presents a 30-year-old woman with palinopsia who had her selective optic radiation lesion assessed by diffusion tensor imaging (DTI). The finding of magnetic resonance imaging (MRI) did not reveal any damage in the selective optic pathway whereas DTI showed a decreased density of the optic radiation tract. Furthermore, the mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were increased in suspected regions of lesions ds, while fractional anisotropy (FA) was decreased. To the authors’ knowledge, this is the first report of a case of palinopsia representing the central nervous system (CNS) tractopathy. Therefore, we encourage further investigation of optic pathway pathology using DTI in complex neurological complications, including palinopsia.
La palinopsia, descrita como la persistencia de imágenes visuales o su reaparición después de que el estímulo haya desaparecido, representa una complicación neuro-oftalmológica rara pero importante. Hasta ahora, la fisiopatología y la principal ubicación de esta condición de mantenimiento visual no se han comprendido de manera clara. Este estudio presenta el caso de una mujer de 30 años con palinopsia, cuya lesión selectiva de radiación óptica fue evaluada mediante imágenes de tensor de difusión (DTI).
La resonancia magnética (MRI) no reveló ningún daño en la vía óptica selectiva, mientras que el DTI mostró una disminución en la densidad del tracto de la radiación óptica. Además, la difusividad media (MD), la difusividad axial (AD) y la difusividad radial (RD) aumentaron en regiones sospechosas de lesiones ds, mientras que la anisotropía fraccional (FA) disminuyó. Según el conocimiento de los autores, este es el primer informe de un caso de palinopsia que representa una tractopatía del sistema nervioso central (CNS); por lo tanto, fomentamos una investigación adicional de la patología de la vía óptica utilizando la DTI en complicaciones neurológicas complejas, incluyendo la palinopsia.
Palinopsia, also known as visual perseveration, is a rare but substantial neuro-ophthalmic condition defined as the recurrence or persistence of visual images after the stimulus is removed.1 A variety range of visual phenomena could be presented in palinopsia leading to the misdiagnosis of this visual preservation with a functional disorder or migraine.1,2 In addition, palinopsia must be distinguished from prevalent physiological after-images induced by a bright visual stimulus.3,4 Structural cerebral lesions as well as tumors, seizures, ischemic, and hemorrhagic cerebrovascular disease have been cited as the main causes of palinopsia.5,6
Previous studies have reported various pathophysiological alterations in both gray and white matter including multifocal white or cortical gray matter lesions, cerebral amyloid angiopathy, and structural and volumic disruption have been attributed to various types of vision changes.7–9 Damage to white matter can have detrimental effects on vision, such bilateral optic neuritis with extensive central nervous system (CNS) white matter lesions in Guillain-Barré syndrome, visual white matter changes in Leber hereditary optic neuropathy and cone-rod dystrophy, disseminated white matter disease in Leber's optic neuropathy, and changes in diffusivity in the optic radiation and fractional anisotropy in the optic nerve and optic tract in patients with amblyopia.10–13 In this study, we report a case of palinopsia in a young female patient with selective optic tract damage, as revealed by DTI. This finding suggests that visual preservation may be a reliable sign of CNS tractopathy.
Case presentationA 30-year-old woman complained of visual impairment in the form of left-sided images remaining in the field of vision (palinopsia). The onset of symptoms progressed gradually and became permanent within two years. The accompanying symptoms were true positional vertigo and left earache. In addition, the patient had a history of occasional headaches without fully meeting migraine and tension criteria.
The patient underwent liposuction and gastric sleeve surgery a month before the onset of symptoms and also she received two injections of the covid-19 vaccine containing an inactivated virus vaccine (Sinopharm) but visual symptoms were aggravated after the second injection.
Clinical findingsThe right side visual acuity (VA) was 8/10, while the left side VA was 9/10 due to refractory errors, and the patient was advised to wear glasses. The Ishihara exam showed that both sides were 14/14.
The relative afferent pupillary defect (RAPD) was bilaterally negative. Furthermore, the lids and their motility were within normal ranges and the deviation was negative. The results of the slit lamp examination were normal and the evaluation of intra-optic pressure (IOP) revealed bilateral abnormalities.
The funduscopy findings indicated a sharp bilateral optic disk margin. In addition, no papilledema was observed on either the right or left side. Also, there were no abnormalities in the other neurological exams.
Diagnostic assessmentThe C30-2 perimetry was repeated several times, and reliable results showed slight depression in the lower environment as well as theintranasal field with no blind spot enlargement on the right and left sides.
An X-ray of the nasal and paranasal sinus demonstrated no abnormalities in the internal ears. Neurosonology showed no signs of atherosclerosis or other vascular injuries.
Optical coherence tomography (OCT), an advanced non-invasive imaging technique that uses light waves to take cross-section pictures of the retina and optic nerve, showed no significant differences on either side. No abnormalities were observed in visual evoked potential (VEP) as an evoked potential caused by a visual stimulus. The video nystagmo graphy (VNG), which measures a type of involuntary eye movement called nystagmus, did not report triggered horizontal nystagmus after irritating the ear and square-like nystagmus in either trial. Electrocochleography (ECOG), measuring electrical potentials generated in the cochlea, revealed endolymphatic hydrops on both sides internal ears. Moreover, no appropriate performances of the inner ear were achieved in response to the caloric test.
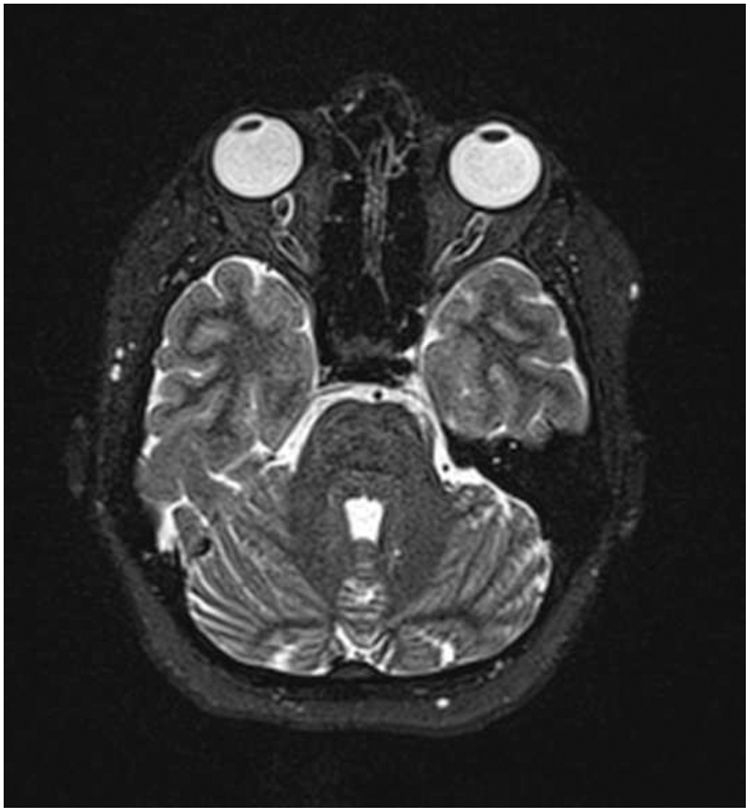
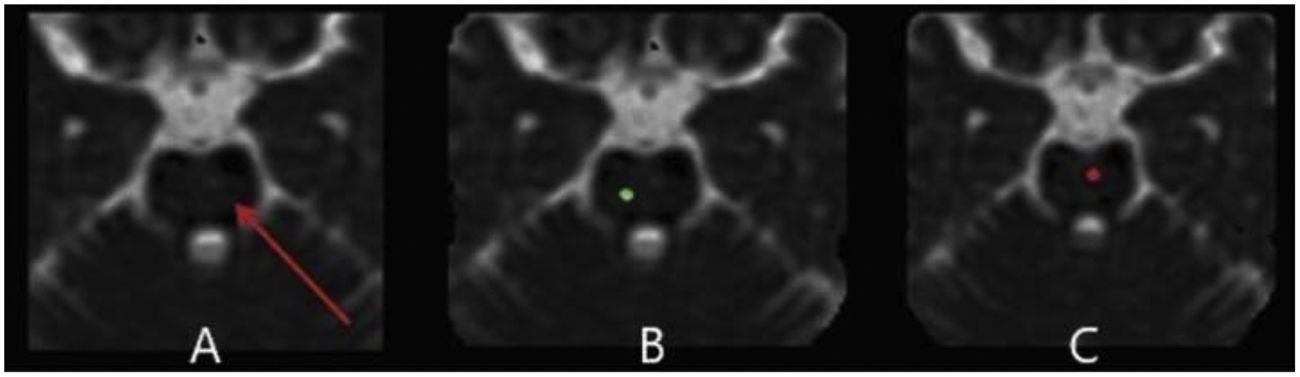
The lumbar puncture (LP) procedure was carried out on the patient. The pressure of cerebrospinal fluid (CSF) was 24cmH2O and the analysis of CSF was normal. The plasma evaluation of toxoplasmosis IgG and IgM was negative. Moreover, there are no abnormalities in vasculitis tests. Routine imaging protocols including a 3 Tesla Siemens scanner for pituitary and brain MRI, MR Venography (MRV) with gadolinium contrast administration, and a 64-channel head-neck coil were performed for the patient. No abnormality was found in the mentioned evaluations (Fig. 1). Except for the suspected lesion region in the left dorsolateral pons with faint changes in signal intensity in the T2FLAIR sequence, a specific investigation with DTI metrics in the suspected lesion region in the left dorsolateral pons was performed. Advanced protocols included three-dimensional (3D) T1-weighted, and DTI for the intent of tractography was performed. Whole-brain 3D T1-weighted images were obtained with the following imaging parameters: TR=1840ms, TE=3.55ms, voxel size=1mm×1mm×1mm, FOV (field of view)=220mm, and slice thickness=1mm with no gap. The DTI image was acquired with a 2D EPI diffusion sequence using the following parameters: TE=79ms, and TR=6200ms, and a total of 64 diffusion sampling directions were selected. The b-value was 2000s/mm.2 The in-plane resolution was 1.08mm. The slice thickness was 2mm. DTI image processing and tractography were performed using DSI studio (http://dsi-studio.labsolver.org). All DTI images were preprocessed and then the diffusion tensor was calculated. First, DTI maps containing fraction anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) were created. Then tractography of the optic radiation was performed using the following pipeline: for this fiber, the anatomy before a tractography atlas was used to perform automatic fiber tracking, a deterministic fiber tracking algorithm was done, and the anisotropy was randomly selected. The angular threshold was randomly selected between 15 and 90 degrees. The step size was 0.43mm. The fiber trajectories were smoothed by averaging the propagation direction with 80% of the previous direction. A seeding region was placed in optic radiation. Tracks with a length of less than 50 or more than 600mm were discarded. A total of 300 tracts were calculated. Topology-informed pruning was applied to the tractography with 16 iteration(s) to remove inappropriate connections.
Magnetic resonance imaging.7 Axial T2-weighted images with fat saturation.
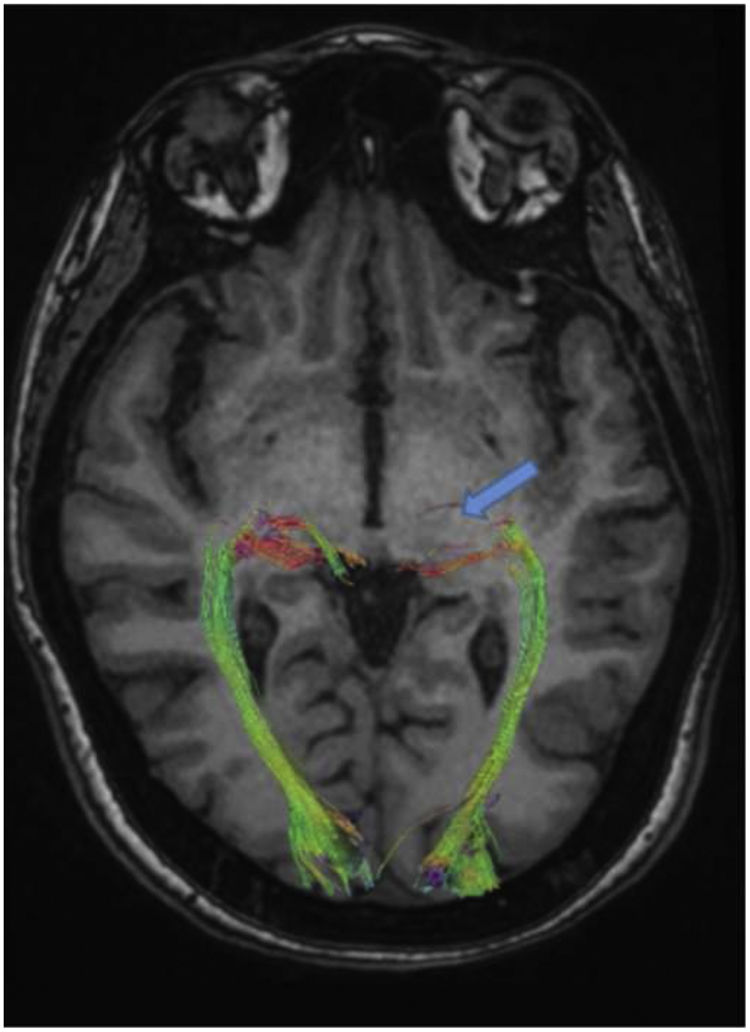
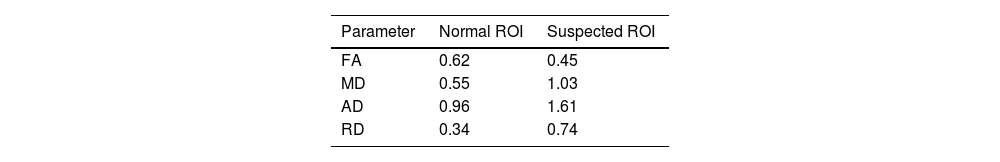
The results of DTI showed that the density of the optic radiation tract was significantly reduced. In addition, in the suspected lesion region in the left dorsolateral pons, the MD map showed increased diffusivity. Furthermore, FA and tract diameter showed significant alterations in the radiation of the patient's left optic nerve (Fig. 2). Moreover, the FA was decreased in areas of suspected lesions in the left dorsolateral pons. The AD and RD in suspected lesions areas of the left dorsolateral pons were increased by 67.71% and 117.65%, respectively, when compared to normal values (Fig. 3), DTI metrics in the suspected lesion areas on pons are shown in Table 1.
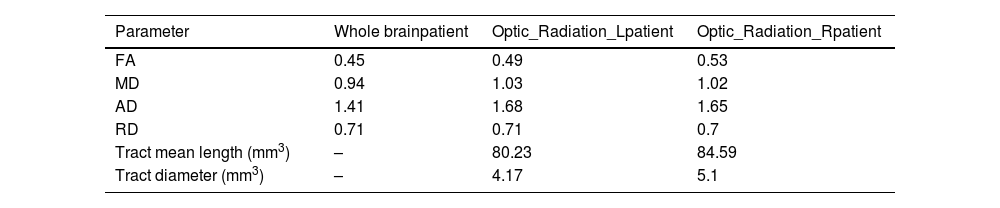
The quantitative DTI results based on the MD map revealed the increased diffusivity in the left optic radiation area. At the same time, the FA map demonstrated that FA is decreased in the related white matter (Table 2).
Diffusion tensor imaging (DTI) metrics in whole brain, right and left optic radiation.
| Parameter | Whole brainpatient | Optic_Radiation_Lpatient | Optic_Radiation_Rpatient |
|---|---|---|---|
| FA | 0.45 | 0.49 | 0.53 |
| MD | 0.94 | 1.03 | 1.02 |
| AD | 1.41 | 1.68 | 1.65 |
| RD | 0.71 | 0.71 | 0.7 |
| Tract mean length (mm3) | – | 80.23 | 84.59 |
| Tract diameter (mm3) | – | 4.17 | 5.1 |
FA: fractional anisotropy; MD: mean diffusivity; AD: axial diffusivity; RD: radial diffusivity.
Based on the CSF pressure of 24cmH2O, the patient received acetylsalicylic acid (ASA) 80mg daily for 6 months and acetazolamide 500 twice daily to alleviate CSF hypertension and reduced CSF production, although no improvement in visual symptoms appeared when CSF pressure became 13cmH2O after repeated LP procedure. In addition, the patient received 6 months of medical treatment with oral diuretics and a low-salt diet for endolymphatic hydrops, which did not improve her visual symptoms. Despite these treatments, the patient continued to experience permanent palinopsia and did not observe any improvement in her visual symptoms.
Follow-up and outcomeThe patient was exposed to 1g of methylprednisolone daily for 3 days after undergoing DTI and observing the results, which indicated tractopathy. Within ten days, their visual symptoms significantly improved. Their visual impairment in the form of persistent images in the field of vision (palinopsia), which was permanent for a period of 2 years, resolved after 10 days of receiving methylprednisolone. Now, they experience palinopsia only 2–3 times a day and only for a few minutes.
DiscussionThe main evidence in this case report is selective CNS tractopathy (optic pathway damage) in a 30-year-old female patient with palinopsia. There are two main categories of palinopsia including hallucinatory and illusory. In hallucinatory palinopsia, the patient observes high-resolution images for a long time that occur anywhere in the visual field. Otherwise, short-lasting low-resolution images that depend on immediate environmental factors are characteristics of illusory palinopsia. Moreover, visual trailing and light streaking are considered other main features of the second type of palinopsia.14–17 Regardless of the type of disease, a physical exam along with neuroimaging and visual field testing are considered the most important methods for definitively diagnosing palinopsia.18,19
The current case report's initial findings show that the optic radiation has changed. The results demonstrated that many of the analyzed optic pathways had higher AD and RD. Previous studies have linked increased AD to higher extracellular water content, followed by changes in axonal water content and atrophy of the white matter fibers. Previous research has linked increased AD to higher extracellular water content, followed by changes in axonal water content and atrophy of white matter fibers.20,21 In addition, AD could be increased with a reduced density of white matter fibers, allowing faster movement of water molecules in an axis parallel to axons,22 whereas alterations in intracellular water induced by impaired axonal transport are followed by reduced AD.23 Along with that RD which reflects diffusivity perpendicular to the axonal fibers and correlate more strongly with myelin abnormalities including an increment in either dysmyelination or demyelination, was also found to be increased in the same segments.24,25 These findings may indicate the atrophy of axonal or fiber along with dysmyelination in several white matter pathways that are involved in vision. In other words, the hypothesis that palinopsia is not just a visual disorder but is a sign of more serious complications could be due to the existence of white matter alterations in both optic radiation and other visual streams, as reported by other authors.5,26,27
Moreover, the FA map of this patient with palinopsia demonstrated decreased FA values in white matter. It is documented that the FA parameter provides reliable information related to fiber integrity and measures the orientation coherence of diffusion. In addition, the negative correlation between FA value and visual parameters of the optic nerves has been suggested.28,29 Importantly, the reduced value of FA is attributed to the process of axonal disruption or loss, demyelination, gliosis, and neuritis.30 In addition to the decreased FA value, this patient had an increased MD value, which is correlated to the height of the optic chiasm and visual field defects.31,32 Besides, increased MD value is considered the neuroimaging signature of white matter degeneration in neurodegenerative diseases including Alzheimer's, and a sensitive biomarker of prodromal cerebrovascular and small vessel diseases.33–35
Additionally, bilateral regional white matter alterations were observed in the large white matter pathways of this patient. This may provide new insights into the localization of changes in the white matter. The white matter lesions were observed in suspected regions in the left dorsolateral pons. Recently, the white matter lesions in the pons have been related to ocular motor abnormalities.36 Also, pathological alterations in pons including lacunar infarcts, which are tiny noncortical infarcts caused by occlusion of a penetrating branch of a large cerebral artery, are reported in patients with palinopsia.18 In this reported patient with palinopsia, our findings implicated both myelin alterations and a larger extracellular space, that could impact the efficacious connectivity between primary visual areas and the thalamus as well as the dorsal, ventral and integrative visual networks, responsible for observed alterations. Similarly, in a patient with visual snow syndrome, a neurological condition described by flickering dots throughout the entire visual field resulted in increased AD and RD in the dorsal visual stream, in the ventral visual stream and the integrative visual stream along with increased values in acoustic and optic radiations as well as in thalamic radiations distal to the thalamus suggested alterations in white matter similar to those seen in our patient with palinopsia.37 In addition, the active demyelination of brain lesions in the optic radiations in the visual pathways and the visually associated region in the temporo-occipital area of the left hemisphere was reported in a case of palinopsia and facial metamorphopsia.38 Moreover, in the patient with palinopsia reported in the present study, the CNS tractopathy revealed symptoms of active demyelination in optic pathways and the Left dorsolateral suspected lesion region in the left dorsolateral pons, too.
It should be noted that the current report is based on a single case, thereby the findings should be interpreted with caution. Currently, there is no comprehensive consensus on the interpretation of DTI-reported alterations. By the consideration of these two concerns, encouraging further studies appears crucially required.
ConclusionThis patient with palinopsia displayed selective optic tract damage associated with vision. We suggest the use of DTI for the assessment of optic pathway pathology in complex neurological complications including palinopsia.
Patient perspectiveThe patient partially recovered, with a reduction in her experience of visual symptoms, specifically palinopsia, after the administration of methylprednisolone. Prior to the steroid treatment, the patient experienced permanent palinopsia throughout the day. However, following the treatment, she reported experiencing palinopsia only 2 or 3 times a day, with each episode lasting only a few minutes. Importantly, the patient was able to continue her work during the day without significant impairment. Furthermore, she was subsequently discharged without receiving maintenance medication. We recommended clinical follow-up examination, brain MRI, and DTI after six months.
Informed consentWritten informed consent was obtained from the patient. It was acknowledged that she was not to be identified via the paper; and that we have anonymized her completely.
Authors’ contributionsEA, VS, ZE, ANM, and MAS have made substantial contributions to conceptualization and design, data curation, investigation, formal analysis, and interpretation of data. EA, VS, and ANM have been involved in methodology, drafting the manuscript, and revising it critically for important intellectual content. EA, and VS have been involved in project administration, resources, software, and supervision. EA, VS, ZE, ANM, and MAS have been involved in validation and visualization.
EA, RH, and ZE have been involved in the writing of the original draft. EA, RH, and ANM have been involved in the review and edition. EA, VS, ZE, ANM, and MAS have given final approval for the version to be published. EA and ZE have been involved in funding acquisition. EA, VS, ZE, ANM, and MAS agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interestThe authors declare that they have no competing interests.