Thirty consecutive multidrug-resistant non-carbapenemase-producing Pseudomonas aeruginosa clinical isolates from patients admitted at a university hospital were studied. Resistance rates to ceftazidime/avibactam, aztreonam/avibactam, ceftolozane/tazobactam and imipenem/relebactam were 40%, 88%, 3%, and 20%, respectively. Ceftazidime/avibactam reverted ceftazidime resistance in 25% of the isolates, whereas imipenem/relebactam did so in 77% of the imipenem-resistant isolates. The analysis of the resistance patterns observed suggests a high contribution of non-enzymatic mechanisms such as efflux pumps and porin alterations.
Se estudiaron 30 aislados clínicos consecutivos de Pseudomonas aeruginosa no productoras de carbapenemasas multirresistentes de pacientes ingresados en un hospital universitario. La resistencia a ceftazidima/avibactam, aztreonam/avibactam, ceftolozano/tazobactam e imipenem/relebactam fue del 40%, 88%, 3% y 20%, respectivamente. Ceftazidima/avibactam revirtió la resistencia a ceftazidima en el 25% de los aislados, mientras que imipenem/relebactam lo hizo en el 77% de los aislados resistentes a imipenem. El análisis de los patrones de resistencia observados sugiere una alta contribución de mecanismos no enzimáticos como bombas de eflujo y alteraciones de porinas.
Pseudomonas aeruginosa (PAE) is an opportunistic gram-negative pathogen and a common cause of nosocomial infections, such as respiratory tract infections, sepsis, surgical wounds and urinary tract infections, among others7. PAE exhibits extended intrinsic resistance to antimicrobial agents and the ability to develop resistance through chromosomal mutations, and/or the acquisition of transferable mechanisms. The resistance profile observed in clinical isolates derives from an interplay of multiple resistance mechanisms, including decreased expression of outer membrane porins, increased production of intrinsic cephalosporinases (PDC), upregulation of efflux pumps, mutations in PBP targets and the presence of β-lactamases (extended-spectrum β-lactamases and/or carbapenemases)10.
PAE is one of the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) that were initially identified as critical multidrug-resistant bacteria (MDR) requiring urgent effective therapies16. The recent introduction of novel β-lactam/β-lactamase inhibitor combinations (BLBLIs), such as ceftolozane/tazobactam, ceftazidime/avibactam, meropenem/vaborbactam or imipenem/relebactam, together with the siderophore cephalosporin cefiderocol, have contributed to mitigating, to some extent, the problem of resistance in PAE9,17.
In 2018, the concept of ‘difficult-to-treat’ resistance (DTR) was proposed. DTR is defined as PAE exhibiting non-susceptibility to piperacillin/tazobactam, ceftazidime, cefepime, aztreonam, meropenem, imipenem–cilastatin, ciprofloxacin and levofloxacin15.
Data from Argentina's National Antimicrobial Resistance Surveillance System (World Health Organization Network, WHONET) show a wavy pattern in the prevalence of third-generation cephalosporin (3GC) and carbapenem resistance from 2013 to 2023. In the 2023 summary of pathogens with critical resistance, a percentage of non-susceptibility of 15.3% to 3GC and 26.1% to carbapenems was reported15.
During the SARS CoV-2 pandemic, in contrast to what was observed in Klebsiella pneumoniae, Acinetobacter baumannii and Enterococcus faecium, no statistically significant increase in the percentages of resistance was observed in the PAE isolates recovered from Hospital de Clínicas José de San Martín, in Buenos Aires city. However, around 15% of the nosocomial PAE isolates showed an antimicrobial resistance profile compatible with the DTR pattern.
The aims of the present study were to evaluate the activity of the new antimicrobials available in our country against clinical PAE isolates with high levels of resistance to agents traditionally used in the treatment of infections caused by this bacterium, and to analyze the associated phenotypic resistance profiles.
Thirty consecutive PAE isolates (non-carbapenemase producers) showing resistance to at least four of the following beta-lactam antibiotics: piperacillin–tazobactam (PT), ceftazidime (CAZ), cefepime (FEP), aztreonam (AZT), meropenem (MER) and imipenem–cilastatin (IMI), from various clinical specimens (excluding intraintestinal samples) from patients admitted to Hospital de Clínicas “José de San Martín” of the University of Buenos Aires between January to July 2024, were included in this study. All isolates included in this study were uniformly resistant to ciprofloxacin (CIP) and levofloxacin (LVX).
These isolates were identified using matrix-assisted laser desorption and ionization time of-flight mass spectrometry (MALDI-TOF MS). The MALDI Biotyper Library v.3.0 and MALDI Biotyper software v 3.1 (Bruker Daltonik GmbH, Bremen, Germany) were used.
Antimicrobial susceptibility was determined using the automated Phoenix 100 ID/AST system (Becton Dickinson Co., Sparks, Md). using the Phoenix NMIC-406 panel according to the manufacturer's recommendations. Ceftazidime/avibactam (CZA), ceftolozane/tazobactam (C/T) and imipenem/relebactam (I/R) susceptibility testing was determined using E-test strips according to the manufacturer's recommendations.
Results were interpreted in accordance with the Clinical and Laboratory Standards Institute (CLSI) breakpoints2. Aztreonam/avibactam (AZA) activity was determined using the agar dilution method in Mueller-Hinton broth (Britania, Buenos Aires, Argentina). Avibactam was used at a fixed concentration of 4μg/ml. Results were interpreted according to CLSI AZT breakpoints2. The presence of a metallobetalactamase was excluded based on the absence of synergy between carbapenems and EDTA in all studied isolates.
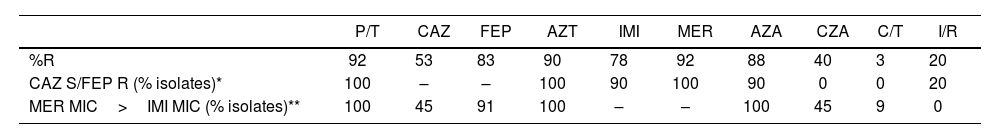
The antimicrobial resistance rates are detailed in Table 1. C/T and I/R showed the highest activity against the PAE isolates. CZA resistance was 40%, higher than what had been reported by Mojica (22%) in a Latin American multicenter study13. Extended-spectrum β-lactamases (ESBL) such as PER and GES, duplication of the residue D149 in OXA-2, mutations in blaPDC, changes in PBPs (PBP3) and MexAB-OprM efflux system overexpression have been associated with CZA resistance13. We observed that CZA reverted CAZ resistance in only 4/16 isolates; in these, CZA MIC values ranged from 4 to 8μg/ml, close to the ECOFF estimated by EUCAST3. These MIC values plus the strong correlation between CZA resistance and AZA MIC values ≥16μg/ml would indicate the presence of non-enzymatic mechanisms of resistance. This observation shows how complex the analysis of resistance mechanisms in PAE can be1. AZA is an antimicrobial combination exhibiting activity against several types of β-lactamases due to the combination of a MBL-stable β-lactam partner with avibactam, which inactivates all serine-type enzymes able to hydrolyze aztreonam8. Lomoskaya has previously reported that an AZA MIC ≥16μg/ml is related to overexpression of the MexAB-OprM efflux pump which affects the activity of all β-lactam agents except IMI, whereas an MIC <8μg/ml correlates with its basal expression11. We observed poor activity of the AZA combination; 80% of the isolates exhibited an AZA MIC ≥16μg/ml, showing the presence of non-enzymatic mechanisms of resistance in most isolates. C/T is a molecular combination between a cephalosporin, ceftolozane, and a β-lactamase inhibitor, tazobactam. Ceftolozane is structurally similar to ceftazidime, despite showing increased stability against AmpC (class C cephalosporinase) β-lactamase-mediated hydrolysis, benefiting from a heavier side chain. The emergence of PAE C/T-resistant isolates due to AmpC overexpression and structural modifications has been recently reported4,12,14. Ceftolozane/tazobactam retained a significant level of activity against PAE with elevated efflux expression, derepressed AmpC or loss of OprD. Our results showed excellent activity of this combination; only one isolate exhibited an MIC: 8μg/ml categorized as having intermediate activity according to CLSI 20242, but was susceptible according to the EUCAST 2024 breakpoints3. The mentioned isolate showed high CAZ, FEP, P/T and CZA MIC values. In this study, C/T demonstrated the highest activity against PAE isolates, differing from data from the region where it was notably lower (susceptibility rates of 68%)5. Additional studies explained that substitutions found in PDC and carbapenemase production were the most common presumed mechanisms of resistance4.
Analysis of antimicrobial susceptibility in the thirty P. aeruginosa isolates.
| P/T | CAZ | FEP | AZT | IMI | MER | AZA | CZA | C/T | I/R | |
|---|---|---|---|---|---|---|---|---|---|---|
| %R | 92 | 53 | 83 | 90 | 78 | 92 | 88 | 40 | 3 | 20 |
| CAZ S/FEP R (% isolates)* | 100 | – | – | 100 | 90 | 100 | 90 | 0 | 0 | 20 |
| MER MIC>IMI MIC (% isolates)** | 100 | 45 | 91 | 100 | – | – | 100 | 45 | 9 | 0 |
AZA: avibactam/aztreonam; AZT: aztreonam; CAZ: ceftazidime; CZA: ceftazidime/avibactam; C/T: ceftolozane/tazobactam; FEP: cefepime; IMI: imipenem; I/R: imipenem/relebactam; MER: meropenem; P/T: piperacillin/tazobactam; R: resistant; S: susceptible.
Imipenem/relebactam is a new antibiotic combination consisting of a carbapenem, imipenem, and a potent non-β-lactam bicyclic diazabicyclooctane β-lactamase inhibitor, relebactam, structurally similar to avibactam with an additional piperidine ring. Mechanisms contributing to resistance to I/R are less clear and generally presumed to be related to the loss of OprD and overexpression of efflux pumps (e.g., MexAB-OprM and/or MexEF-OprN)4. We observed five isolates with MIC values: 4/4μg/ml (categorized as intermediate by CLSI 20242, and resistant by EUCAST 2024)3,14. These results are similar to the ones published by Poirel in non-carbapenemase-producing Pseudomonas spp9. It is noteworthy that different studies have demonstrated a good correlation between the methodology used in the present work and the broth dilution method6. In our knowledge, there have been no previous national results evaluating the I/R activity in PAE isolates.
As previously mentioned, the multiple intrinsic and acquired mechanisms together with their level of expression hinder the possibility to establish their presence in these isolates and to define their predominant role in determining the resistance to one antimicrobial agent7,8. Due to this limitation, we evaluated the activity of the new antimicrobials against the two most frequently observed antibiotic profiles: susceptibility to CAZ together with FEP resistance and MER MIC>IMI MIC (Table 1).
Activity dissociation between CAZ and FEP was observed in 10 isolates, which could be related to the presence of β-lactamases and/or non-enzymatic mechanisms. Ninety percent of the isolates exhibited AZA MIC values ≥16μg/ml, showing the presence of non-enzymatic mechanisms within this susceptibility profile; the same analysis applies to isolates showing MIC values for MER higher than those for IMI: the absence of reversion of CAZ resistance when adding AZA also suggests the presence of non-enzymatic mechanisms.
In conclusion, in this study we report the resistance profiles to the most frequently used antimicrobial agents in the treatment of infections caused by MDR PAE isolates. We consider that although the results presented here are restricted to a single medical center, the fact that they differ from previously published data in our region highlights the need for periodic monitoring of antimicrobial resistance in this bacterium.
FundingThis study was partially supported by the UBACYT project (UBACYT 20020220300060BA Famiglietti A-Rodriguez CH).
Conflict of interestAll authors declare they have no conflict of interest.