Listeria monocytogenes is one of the most important bacteria associated with foodborne diseases, soft cheese being an important L. monocytogenes vehicle. In Ecuador, soft cheese is consumed in 84.3% of urban households. We determined the prevalence of L. monocytogenes and serogroups in 260 fresh artisanal soft cheese samples collected in 18 of 24 Ecuadorian provinces. Listeria spp. detection was carried out by culture-dependent and independent methods; 14.23% of samples were positive for L. monocytogenes. Serogroup IVb was found in 83.78% of the food isolates. Serogroups IIb and IIa were present in 8.11% of our isolates. To our knowledge, this is the first report of L. monocytogenes serogroups associated with food in Ecuador; we also found serogroup similarities between cheese isolates and clinical isolates.
Listeria monocytogenes es una de las bacterias más importantes asociadas con enfermedades transmitidas por alimentos, y el queso fresco es un importante vehículo de transmisión de L. monocytogenes. En Ecuador, el consumo de quesos frescos se produce en el 84,3% de los hogares urbanos. Determinamos la prevalencia de L. monocytogenes y sus serogrupos en 260 muestras de queso fresco artesanal recolectadas en 18 de las 24 provincias ecuatorianas. La detección de Listeria spp. se llevó a cabo mediante métodos independientes y dependientes de cultivo; el 14,23% de las muestras fueron positivas para L. monocytogenes. El serogrupo IVb se encontró en el 83,78% de los aislados de alimentos y los serogrupos IIb y IIa estaban presentes en el 8,11%. Hasta donde sabemos, este es el primer informe de serogrupos de L. monocytogenes asociados a alimentos en Ecuador; también encontramos similitudes de serogrupo entre aislados de queso y aislados clínicos.
Foodborne diseases (FBDs) are still a major public health concern worldwide, despite the strict controls implemented in the food industry. Each year approximately 600 million illnesses and 420000 deaths occur because of FBDs such as norovirus infections, salmonellosis, campylobacteriosis, listeriosis, and other enteric diseases particularly in developing regions14.
Among the most important FBDs, listeriosis, caused by Listeria monocytogenes, is associated with severe complications such as abortion, meningitis, septicemia, encephalitis, with a high mortality rate10. L. monocytogenes is a facultative intracellular pathogen, widely distributed in the environment, with the ability to prosper at refrigeration temperatures8. Listeria vehicles are ready-to-eat (RTE) foods including meat products, vegetables, and with particular interest, milk and dairy products8.
Thirteen serotypes, distributed among 4 lineages, had been described worldwide; however, three of them, 1/2a, 1/2b and 4b are more commonly associated with human listeriosis (presented both as outbreaks and as sporadic cases)8,10. Serotype 4b is the most frequent, being responsible for 50% of the large epidemic outbreaks reported and 90–95% of sporadic cases8.
In Ecuador, the impact of listeriosis on public health is unknown because it is not a notifiable disease despite the severity of the symptoms. L. monocytogenes has been detected by molecular and culture methods, in food such as soft cheese, sausages, and yogurt, and even on surfaces of food factories. However, these studies detected only bacterial DNA and not necessarily viable microorganisms; or identified the bacteria at the genus level1,2,11,12, which is a limitation because L. monocytogenes is the only species associated with human disease10.
In this study, we determined the prevalence of L. monocytogenes in 260 samples of artisanal soft cheeses since these dairy products are consumed in 84.3% of urban and rural households. Small farms from remote locations produce cheese to avoid milk spoilage usually using rudimentary methods. The samples were collected from March to September, 2018 in street markets of 18 out of 24 provinces of Ecuador (Table 1). The cheeses included in our study did not have a sanitary registration certificate issued by the National Authority. The detection was performed by culture dependent and independent methods. We also compared serogroups isolated from cheeses with clinical isolates from June 2015 to July 2018 provided by the Laboratory for Antibiotic Resistance of the National Institute of Public Health Research of Ecuador.
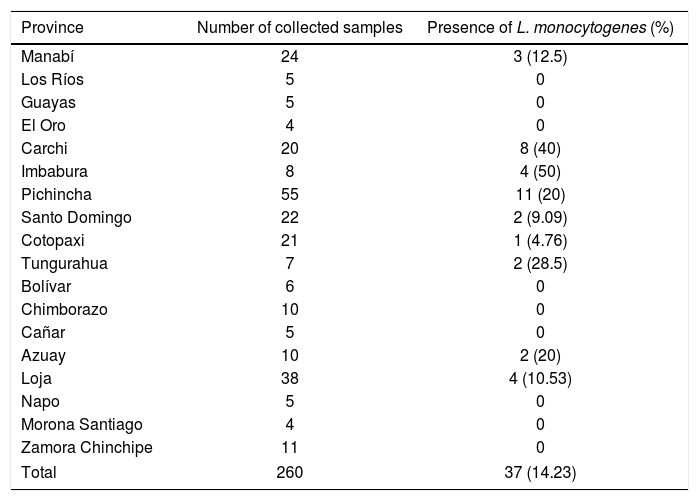
Distribution of L. monocytogenes culture-positive samples in 18 Ecuadorian provinces.
| Province | Number of collected samples | Presence of L. monocytogenes (%) |
|---|---|---|
| Manabí | 24 | 3 (12.5) |
| Los Ríos | 5 | 0 |
| Guayas | 5 | 0 |
| El Oro | 4 | 0 |
| Carchi | 20 | 8 (40) |
| Imbabura | 8 | 4 (50) |
| Pichincha | 55 | 11 (20) |
| Santo Domingo | 22 | 2 (9.09) |
| Cotopaxi | 21 | 1 (4.76) |
| Tungurahua | 7 | 2 (28.5) |
| Bolívar | 6 | 0 |
| Chimborazo | 10 | 0 |
| Cañar | 5 | 0 |
| Azuay | 10 | 2 (20) |
| Loja | 38 | 4 (10.53) |
| Napo | 5 | 0 |
| Morona Santiago | 4 | 0 |
| Zamora Chinchipe | 11 | 0 |
| Total | 260 | 37 (14.23) |
Following the manufacturer's instructions of the Molecular Detection System (MDS 3M™), 25g of each sample were pre-enriched with 225ml of 3M Demi-Fraser broth with 5% ferric ammonium citrate. After pre-enrichment, samples were analyzed with the MDS instrument and kit for L. monocytogenes (3M™).
All the positive samples by MDS 3M™ were cultured in esculin-based Palcam Listeria Selective Agar (BD™) for 24h at 37°C, for the isolation and detection of L. monocytogenes and other Listeria species. Two isolates per sample were recovered and stored at −80°C in skim milk with 10% glycerol for further molecular analysis.
From pure culture, 4–5 colonies were pooled and total genomic DNA was extracted from all Listeria isolates using DNAzol (Invitrogen™ Carlsbad, USA). Additionally, we extracted DNA from 20 clinical isolates using Pure Link Genomic Mini Kit (Invitrogen™, Carlsbad, USA).
For L. monocytogenes detection, PCR amplification reactions were carried out following the standardized protocols described elsewhere13. The serogroup detection was performed by Doumith et al.5 Four molecular serogroups can be identified by this technique: IIa (serotypes 1/2a, 3a); IIb (serotypes 1/2b, 3b and 7); IIc (serotypes 1/2c and 3c); and IVb (serotypes 4b, 4d and 4e). DNA from L. monocytogenes 4b ATCC 13932 was used as positive control for both analyses, and nuclease-free water as a negative control.
The antimicrobial susceptibility to different antibiotics was tested following the CLSI M45 guidelines for Fastidious Bacteria (2015)3 and EUCAST 20186. Test for penicillin, ampicillin, erythromycin, trimethoprim/sulfamethoxazole, and meropenem was obtained by the broth microdilution technique, using panels of lyophilized antibiotics for Gram-positive bacteria (Sensititre GPALL1F, Thermo Scientific®). In addition, meropenem (10μg/disk) was tested by the disk diffusion method using Mueller Hinton agar supplemented with 5% of horse blood. The addition of β-NAD to Mueller Hinton agar is recommended when testing sulfonamides and trimethoprim, therefore the reagent was not added for the meropenem disk diffusion. Streptococcus pneumoniae ATCC 49619 was used for quality control.
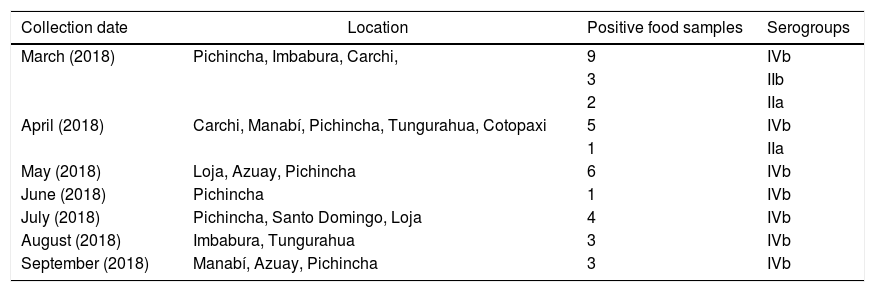
Of the 260 food isolates, 37 (14.23%) were positive for L. monocytogenes (molecular analyses); 31 isolates (83.78%) belonged to serogroup IVb; 3 (8.11%) to serogroup IIa, and 3 (8.11%) to serogroup IIb (Table 2).
Serogroups of L. monocytogenes isolated from soft cheese in different Ecuadorian provinces.
| Collection date | Location | Positive food samples | Serogroups |
|---|---|---|---|
| March (2018) | Pichincha, Imbabura, Carchi, | 9 | IVb |
| 3 | IIb | ||
| 2 | IIa | ||
| April (2018) | Carchi, Manabí, Pichincha, Tungurahua, Cotopaxi | 5 | IVb |
| 1 | IIa | ||
| May (2018) | Loja, Azuay, Pichincha | 6 | IVb |
| June (2018) | Pichincha | 1 | IVb |
| July (2018) | Pichincha, Santo Domingo, Loja | 4 | IVb |
| August (2018) | Imbabura, Tungurahua | 3 | IVb |
| September (2018) | Manabí, Azuay, Pichincha | 3 | IVb |
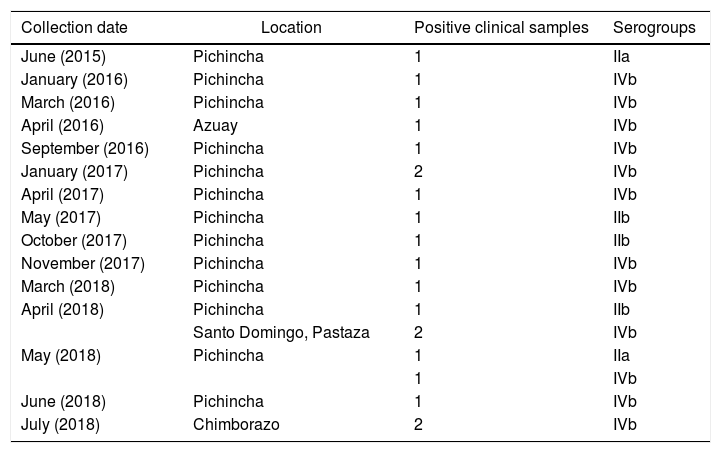
Of the clinical isolates, 15 (75%) belonged to serogroup IVb; 2 (10%) to serogroup IIa; and 3 (15%) to serogroup IIb (Table 3). Several studies have concluded that cases of listeriosis are commonly associated with serotypes 4b, 1/2a and 1/2b (serogroups IVb, IIa, and Iib, respectively), which are considered the most pathogenic8,10. Serotype 4b (serogroup IVb) has been found to be responsible for 50% of the clinical cases and 70% of outbreaks worldwide8. Strains from this serotype replicate better than those from other serotypes in monocytes/macrophages. This feature may be involved in their pathogenicity, particularly in systemic dissemination7. Other serogroups found in this study were IIb (8.11%), and IIa (8,11%) (Table 3). Serotypes 3a, 3b, 3c, 4a, 4c, 4e, 4d and 7 are uncommonly found in food and rarely associated with human infection13.
Serogroups of L. monocytogenes from associated clinical samples and geographic origin.
| Collection date | Location | Positive clinical samples | Serogroups |
|---|---|---|---|
| June (2015) | Pichincha | 1 | IIa |
| January (2016) | Pichincha | 1 | IVb |
| March (2016) | Pichincha | 1 | IVb |
| April (2016) | Azuay | 1 | IVb |
| September (2016) | Pichincha | 1 | IVb |
| January (2017) | Pichincha | 2 | IVb |
| April (2017) | Pichincha | 1 | IVb |
| May (2017) | Pichincha | 1 | IIb |
| October (2017) | Pichincha | 1 | IIb |
| November (2017) | Pichincha | 1 | IVb |
| March (2018) | Pichincha | 1 | IVb |
| April (2018) | Pichincha | 1 | IIb |
| Santo Domingo, Pastaza | 2 | IVb | |
| May (2018) | Pichincha | 1 | IIa |
| 1 | IVb | ||
| June (2018) | Pichincha | 1 | IVb |
| July (2018) | Chimborazo | 2 | IVb |
We found L. monocytogenes in 14.23% of the cheese samples, which is similar to the prevalence found in cheeses in Brazil4 but lower than the occurrence reported in Colombia9.
Two provinces (Pichincha and Carchi) showed the highest prevalence of L. monocytogenes in cheeses (Table 2). The province of Pichincha exhibited the highest number of clinical cases of listeriosis (Table 3), also showing better medical and surveillance services than other provinces.
With regard to other Listeria species, we found L. innocua (80%), L. welshimeri (10%) and L. grayi (10%). L. monocytogenes can coexist with competing microorganisms and other Listeria species. L. innocua is the closest species to L. monocytogenes, and both share the same ecological niche8. The presence of other Listeria spp. such as L. welshimeri and L. grayi, found in cheeses, may be associated with inadequate processing, storage, or transportation of these products.
In reference to antimicrobial susceptibility, all strains in this study were sensitive to all the antibiotics analyzed: trimethoprim/sulphamethoxazole, penicillin, ampicillin, erythromycin and meropenem. These results suggest that antibiotic treatment may adequately work in listeriosis cases in Ecuador.
To the best of our knowledge, this study is the first report of viable L. monocytogenes in food and clinical samples, and also the first to identify serogroups in Ecuador. The presence of L. monocytogenes in our samples suggests the need to demand thermal treatments in cheese processing as well as better epidemiological surveillance of the pathogen and the disease in our country.
FundingThis work was supported by a mini grant from COCIBA, Universidad San Francisco de Quito, Ecuador (Project 7707).
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors acknowledge Juan Mosquera for valuable comments during the manuscript preparation. We also thank Enterprise 3M and its business coordinator, Diego Ortiz, for their support and material donation. We also acknowledge Cecilia Palacios for providing a positive control for L. monocytogenes.