Rachiplusia nu (Guenée) (Lepidoptera: Noctuidae) is one of the major lepidopteran pests defoliating soybeans (Glycine max Merrill) in Argentina. The combined use of chemical insecticides and entomopathogenic fungi is a promising pest-control option to minimize adverse chemical effects. In this work, we evaluated the interactions between five insecticides—two being considered biorational—and five fungal entomopathogenic strains under laboratory conditions in order to determine the possible usefulness of combinations of these agents against R. nu. The insecticides were tested for compatibility at four doses by in vitro bioassay and for the lethality of R. nu by inoculations at three doses. Fungal strains were applied at 1×108, 1×106, and 1×104conidia/ml. The combinations of those insecticides with Beauveria bassiana (LPSc 1067, LPSc 1082, LPSc 1098), Metarhizium anisopliae (LPSc 907), and Metarhizium robertsii (LPSc 963) caused higher R. nu–larval mortalities than any of the individual agents alone. We observed significant differences in the in vitro conidial viability, vegetative growth, and conidia production of the five strains of entomopathogenic fungi exposed to different doses of the chemical insecticides. The combination gamma-cyhalothrin–LPSc-1067 caused the highest percent mortality of R. nu larvae, with synergism occurring between the two agents at 50% and 25% of the maximum field doses.
Rachiplusia nu (Guenée) (Lepidoptera: Noctuidae) es una de las principales plagas de lepidópteros defoliadores del cultivo de soja (Glycine max Merrill) en Argentina. El uso combinado de insecticidas químicos y hongos entomopatógenos es una opción de control de plagas prometedora para minimizar los efectos químicos adversos. En este trabajo se evaluaron las interacciones entre 5 insecticidas —2 de ellos considerados biorracionales— y 5 cepas fúngicas entomopatógenas en condiciones de laboratorio, para determinar la posible utilidad de combinaciones de estos agentes frente a R. nu. Se evaluó la compatibilidad de los insecticidas a 4 dosis mediante bioensayos in vitro y la letalidad de aquellos sobre R. nu mediante inoculaciones a 3 dosis. Las cepas fúngicas se evaluaron a concentraciones de 1×108, 1×106 y 1×104conidios/ml. Las combinaciones de estos insecticidas con Beauveria bassiana (LPSc 1067, LPSc 1082, LPSc 1098), Metarhizium anisopliae (LPSc 907) y Metarhizium robertsii (LPSc 963) causaron una mayor mortalidad de larvas de R. nu que cualquiera de los agentes individuales. Asimismo, se observaron diferencias significativas en la viabilidad de los conidios in vitro, el crecimiento vegetativo y la producción de conidios de las 5 cepas de hongos entomopatógenos expuestos a diferentes dosis de los insecticidas químicos. La combinación gamma-cialotrina-LPSc-1067 causó el mayor porcentaje de mortalidad de larvas de R. nu, con un efecto de sinergismo entre los 2 agentes al 50 y el 25% de las dosis de campo recomendadas por el fabricante.
Oilseeds and cereals are excellent field crops in Argentina, as they provide a major portion of the income and a high percentage of the food base for the country's population. The soybean—the main oilseed crop of Argentina—is also the most widespread, with a planted area of 20 million hectares and an annual production of 53000000 metric tons, equivalent to a value of 26 billion US dollars22. In the last 21 years, the total area planted with soybeans has increased 3.6-fold and the production 4.8-fold16. Argentina, for its part, is the third largest producer of soybeans in the world after the USA and Brazil and the first exporter of oil and soybean meal22. One of the main causes of decreasing in the average annual yields has been attributed to the attack of crops by insect pests. Agricultural crops are affected by a wide variety of insects, and in Argentina, at the year 2013; soybean crop losses caused by insect pests were computed as 10% of total annual production. The amount of chemical insecticide required for control pests was 30 million of kilograms valued in 260 US million dollars7.
Rachiplusia nu—a widespread species on the South-American continent—is particularly common in Argentina, Bolivia, Brazil, Chile, and Uruguay. The larval stage of R. nu can cause severe damage to crops including soybeans, sunflowers (Helianthus annuus L.), corn (Zea mays L.), wheat (Triticum sp L.), and alfalfa (Medicago sativa L.). In Argentina, this insect represents major pests of soybean crops. The pest infestations usually start from December and reach maximum levels in January and February16. The early instars larvae of R. nu feed on leaf parenchyma without damaging the leaf veins, reaching their highest voracity during their fifth larval instar, eating during their larval period, between 100 and 110cm2 of soybean leaves. This decrease in leaf area, results in lower sunlight interception, lower photosynthetic capacity, loss of material stored in leaf and shortening the period of grain filling31. To date, farmers use broad-spectrum insecticides to combat this pest, but the indiscriminate use of these chemical agents is problematic and undesirable because of their poor selectivity, residual toxicity in the environment, bioaccumulation, and high frequency of resistance formation in insects13. According to the Food and Agriculture Organization of the United States9, a comprehensive public policy is essential for plant health. Five major aspects of today's social and economic reality in Argentina are1: the decisive role in plant health requirements for international trade2; the urgency of the need for the quality control of food safety in the diet, especially within the context of the exportation of foods3; the need for significant improvements in the safety of rural agricultural workers4; the environmental quality of the rural and small urban settlements; and finally5 the conservation of natural resources, especially water.
For these reasons, the biologic control of insect pests through the use of pathogens, parasites, and predators has received particular attention in recent years. Among these microorganisms, the role of entomopathogenic fungi as prominent biologic-control agents has been the subject of intense research for over 100 years34. The fungal species Beauveria bassiana (Bals.-Vuill.) Vuillemin and Metarhizium anisopliae (Metschn.) Sorokin are the most commonly used for the biologic control of the insect pests of agricultural crops18,25. Moreover, less used in biological control, but not least, is the case of insect-pathogenic fungus, Metarhizium robertsii J.F. Bisch., Rehner & Humber. It is a common inhabitant of soils worldwide3 and has been studied and used as an insect pathogen for biocontrol15,20. A disadvantage, however, of using entomopathogenic fungi in pest-control programs for field crops is the excessive time required to kill the target insect20. For this reason, the combined use of entomopathogenic fungi plus sublethal doses of chemical insecticides has been found to be a promising alternative for reducing the amount of chemicals used for pest control, thus protecting the health of the environment and the agricultural workers5.
In this study, we evaluated, under laboratory conditions, the interactions between five insecticides, two being considered bio rational, and five strains of entomopathogenic fungi in order to determine the possible usefulness of combinations of these agents against the soybean-defoliating lepidopteran pest R. nu.
Materials and methodsInsect rearingThe R. nu individuals used in the toxicity tests were obtained from a colony (provided by the company AgIdea [Agricultural Innovation Applied Research]) without history of insecticide exposure. The larvae were kept on a semi synthetic diet14 in a controlled chamber at 25±0.5°C, a 75% relative humidity, and a light/dark photoperiod of 16/8h, during the trial time.
Fungal strainsThe entomopathogenic fungi used in this study were obtained from Spegazzini Institute culture collection, LPSc 1067 (GenBank accession number KF500409), LPSc 1082 (GenBank accession number KJ7722495) and LPSc 1098 of B. bassiana, LPSc 907 (GenBank accession number KJ772494) of M. anisopliae and LPSc 963 of M. robertsii J.F. Bisch., Rehner & Humber. All isolates were preserved by freeze drying (lyophilization) technique. The provisional species identification (LPSc 1098 and LPSc 963) by morphology was corroborated by genetic analysis after extracting the DNA of the monosporic cultures according to Stenglein and Balatti33. Polymerase-chain reactions (PCRs) were carried out in an XP thermal cycler (Bioer Technology Co, Hangzhou, China) to amplify and sequence the internal-transcribed-spacer rDNA region of B. bassiana through the use of the primer pairs ITS5/ITS430 and the translation-elongation factor 1-alpha (the elongation-factor–intron sequence) of M. anisopliae by means of the primer pairs EF1T/EF2T4. Fragment similarities to previously published sequence data were examined by BLASTn1 in the web page of the National Center for Biotechnology Information. The sequences generated in this study were submitted to GenBank (accession numbers KT163259 for B. bassiana LPSc 1098; and KT163258 for M. robertsii LPSc 963).
BLAST searches of the LPSc 1098 showed 100% of similarity (e-value 0.0) with B. bassiana (e.g., accession numbers KU726564, KU158472, KT443982) and for LPSc 963 sequence showed 100% of similarity (e-value=0.0) with several sequences of M. robertsii (e.g., accession numbers KT862515, KR70649, KP027978).
The choice of these fungal strains was based on their laboratory efficacy against pest grasshopper and locust species from Argentina27,28. The conidia of the fungal strains obtained from cultures on potato-dextrose-agar (PDA) medium (Britania S.A., Buenos Aires, Argentina) after incubation for 10 days at 25°C in the dark were harvested with disposable cell scrapers (Fisherbrand™) and placed in test tubes containing 0.01% (v/v) polyoxyethylene sorbitan monolaurate (Tween 80™; Merck). The resulting suspensions were vortexed for 2min, filtered through four layers of sterile muslin, and adjusted to 1×108, 1×106, and 1×104conidia/ml after cell counting in a Neubauer hemocytometer. The viability of the conidia from each isolate and the concentrations used in the tests were determined after 24h as described by Goettel and Inglis12. This germination test was repeated for each stock suspension to maintain the constancy of the viability assessments. In all instances, the average viability of the conidia was over 95%.
InsecticidesThe synthetic chemical pesticides Archer Plus™ (gamma-cyhalothrin 15% [w/v], Cheminova SA, Argentina), Lambda™ (lambda-cyhalothrin 25% [w/v], Handelsgesellschaft Detlef Von Appen, Argentina), and Coragen™ (rynaxypyr 20% [w/v], DuPont S.A., Argentina), along with two biorational chemical insecticides, Match™ (luphenuron 5% [w/v], Sygenta S.A., Argentina) and Intrepid™ (methoxyfenozide 24% [w/v], Dow AgroSciences S.A., Argentina) were used in these experiments.
The insecticides were tested for fungal compatibility at four doses by in vitro bioassay: twice the average concentration recommended for field application on soybean (200%), the average recommended (100%), 50% of the average, and finally 25%; to give levels of 104, 52, 26, and 13ppm (mg active ingredient/l [a.i./l]) for gamma-cyhalothrin; 174, 87, 44, and 22ppm (mg a.i./l) for lambda-cyhalothrin; 120, 60, 30, and 15ppm (mg a.i./l) for rynaxypyr; 200, 100, 50, and 25ppm (mg a.i./l) for luphenuron; and 288, 144, 72, and 36ppm (mg a.i./l) for methoxyfenozide7, and at three doses in the experimental inoculations against R. nu: the average recommended for field application on soybean (100%), 50% of the average, and finally 25%; to give levels.
In vitro compatibility of fungal isolates with chemical insecticidesAssessment of germinationThe conidial viability of was measured as described by Goettel and Inglis12. A suspension of 1×106conidia/ml in Tween 80™ was vortexed for 2min, then filtered through four layers of sterile muslin. Of this suspension, 1ml was combined with 1ml of each of the four doses of insecticides indicated in the previous section and then incubated for 1h at room temperature. Next, 800μl of sterile PDA culture medium was layered onto each of a series of autoclaved microscope slides at a thickness of about 2mm and the slides transferred to Petri dishes containing a filter-paper disk. To each of those dishes, 0.5ml of one of the fungus-insecticide admixtures was spread over the agar-coated slide. The dishes were then incubated at 25°C for a single 12/12-h photoperiod and the percent germinated conidia quantified 24h later. A control without the addition of chemical insecticide was made for each fungal isolate. The conidia were considered to have germinated once the germ tube had reached half a conidium's length. Each assessment was made in triplicate per treatment and 300 conidia counted under each experimental condition. The data were analyzed through the use of the generalized linear model with the software GenStat Twelfth Edition11, with a binomial error distribution and logit-link function. The means were compared according to the least significant difference (LSD; p≤0.05).
Assessment of vegetative growth and conidia productionThe chemical insecticides were incorporated into the PDA medium supplemented with streptomycin (0.5g/l), at 45±5°C, and poured into Petri dishes at room temperature as described by Neves et al.23 After medium solidification, the fungi were inoculated with a sterile punch of 1cm2 in the center of the Petri dish, (at five dishes per treatment). The dishes were then incubated at 25±1°C for a 12/12-h photoperiod. The colony areas were finally measured on vegetable paper with a planimeter at 7 days for B. bassiana (LPSc 1067, LPSc 1082, LPSc 1098) and 10 days for M. anisopliae (LPSc 907) and M. robertsii (LPSc 963) after the inoculation. Once the areas of the colonies were drawn, 10 disks (2cm2) from the center of the colony in each treatment were collected for the quantification of conidia production. Each disk was placed in a glass tube and the conidia suspended in 10ml of 0.01% (v/v) Tween 80™ and counted in a Neubauer chamber. The data were analyzed according to the generalized linear model with the software GenStat Twelfth Edition11, with a logarithmic Poisson-distribution function for conidia production and a normal distribution function for vegetative growth. The means were compared by LSD (p≤0.05).
Experimental inoculationsExperimental inoculations were conducted on R. nu third-instar larvae in order to determine the percent mortality at each dose of the insecticide (100, 50 and 25% the average field recommended) and at different concentration of fungal strains (1×108, 1×106, and 1×104conidia/ml). Each larva was sprayed with 300μl of each treatment through the use of a glass fine mist sprayer (discharge rate 0.10±0.02ml). The larvae were inoculated individually and then placed in sterile containers (30cm3) with an artificial food available ad libitum. Three replicates and one control of 10 individuals each was used with each of the treatments. The larval controls were sprayed in the same mode, but with the vehicle 0.01% [v/v] Tween 80™ alone. Both the treated and the control insects were maintained at 25±0.5°C, a 75% relative humidity, and a light/dark photoperiod of 16/8h. The cumulative mortality was recorded daily for 10 days. The dead larvae with no external mycelia were surface-sterilized by successive immersions in 70% ethanol (10–15s), 0.5% (w/v) sodium hypochlorite solution (1min), and sterile distilled water (1min, two consecutive dips) according to Lacey and Brooks19. Next, the specimens were placed in sterile Petri dishes (60mm diameter) with filter-paper disks periodically moistened with sterile distilled water and the dishes incubated at 25°C in the dark for 3–5 days for fungal development. Mycosis was confirmed by microscopical examination of the dead larvae. When the mortality in the treated larvae at different concentrations of the five fungal strains and different dose of chemical compounds was 50% or higher, the median survival time (MST) in days were calculated based on the Kaplan–Meier survival-distribution function35. Pairwise comparisons between the survival curves were made by the Wilcoxon test. Moreover, when the mortality was 50% or higher in the larvae treated with the different chemical compounds alone, the following lethal and sublethal-toxicity parameters were calculated (DEBtox v. 2.0.1 program)17,29 the median lethal concentration (LC50), the concentration lethal to 10% of the exposed individuals (LC10), and the no-effect concentration (NEC). Significant differences between the percent mortalities after a 10-day exposure to the different treatments were analyzed according to the generalized linear model with a binomial distribution and logit-link function. The means were compared by the LSD test (p≤0.05).
Interaction between entomopathogenic fungi and chemical insecticides against R. nuThe interaction of B. bassiana (LPSc 1067, LPSc 1082, LPSc 1098), M. anisopliae (LPSc 907) and M. robertsii (LPSc 963) with the insecticides gamma-cyhalothrin, lambda-cyhalothrin, rynaxypyr, luphenuron, and methoxyfenozide against R. nu third-instar larvae was investigated in the following manner. Of each fungal suspension and each insecticide solution, 5ml were combined, to give a final volume of 10ml (these were adjusted to reach the abovementioned concentration for each fungal strain and chemical insecticide evaluated). Suspensions were homogenized by vortexing for 2min. The experimental design and inoculation techniques were performed as indicated in the previous section. The cumulative mortality was recorded daily for 10 days and mycosis confirmed by microscopical examination of the dead larvae. When the mortality reached 50% or higher, the toxicologic effect of gamma-cyhalothrin, lambda-cyhalothrin, rynaxypyr, luphenuron, and methoxyfenozide on those R. nu third-instar larvae were calculated. The LC10–LC50 and NEC values were expressed as ppm, and the MST in days, both parameters along with the respective standard deviations (SDs). Significant differences between the percent mortalities after a 10-day exposure to the different pesticide combinations were analyzed according to the generalized linear model with a binomial distribution and logit-link function. The means were compared by the LSD test (p≤0.05).
The determination of additive, synergistic, or antagonistic interaction was based on a binomial test involving a comparison of the expected and observed percentage mortalities as described by Nishimatsu and Jackson24. The expected mortality at a set concentration of insecticide and fungal strain was based on the formula Pe=Po+(1−Po)(P1)+(1−Po)(1−P1)(P2), where Pe is the expected mortality on combination of the 2 insecticidal agents, Po the natural (control) mortality, P1 the mortality after treatment with the insecticide alone, and P2 the mortality, after treatment with the fungal strain alone. Additivity was indicated if χ2<3.84. Antagonism was indicated if χ2>3.84 and Pc<Pe, where Pc is the observed mortality of the insecticide and fungal-strain combination. Synergism was indicated if χ2>3.84 and Pc>Pe.
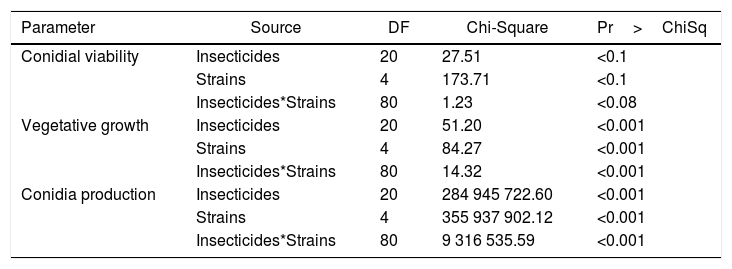
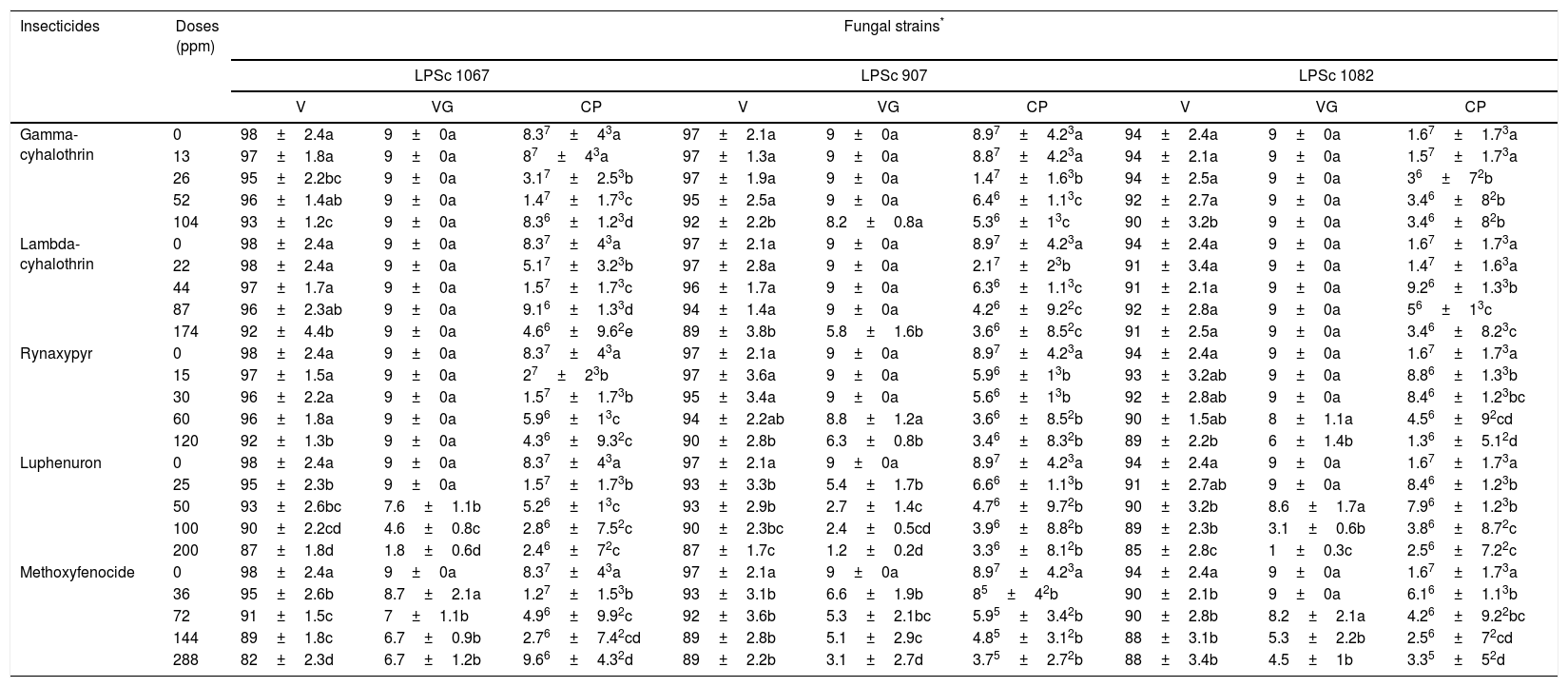
ResultsWe observed significant differences in the in vitro conidial-viability test among the different insecticides and among the five fungal strains alone as well as in the interaction between the insecticides and the strains (hereafter, referred to as insecticides*strains) as defined by a p≤0.083 (Table 1). Reductions occurred in the percent conidial germination in all the fungal isolates with increased doses of the chemical insecticides: thus, a greater decrease occurred when the dose of insecticide was twice that recommended for field application by the manufacturer (Table 2). The insecticides that less affected the conidial germination of the different isolates were gamma- and lambda-cyhalothrin (Table 2). Significant differences likewise obtained in the vegetative growth of the fungi among the different fungal strains either alone or exposed to the five chemical insecticides (Table 1). The most significant reduction was observed in the colony area for the B. bassiana strains LPSc 1082, LPSc 1098 and M. anisopliae strain LPSc 907, when grown on PDA medium at a dose of 200ppm luphenuron. In contrast, the vegetative growth of the strain LPSc 1067 was not affected on the same medium supplemented with gamma-cyhalothrin, lambda-cyhalothrin, and rynaxapyr; even at the highest doses used of 104, 174, and 120ppm, respectively; while the colony area of the fungal strain LPSc 907 was likewise unchanged by gamma-cyhalothrin (Table 2). In a similar manner, the vegetative growth of LPSc 963 remained constant in the presence of methoxyfenocide and that of LPSc 1082 and LPSc 1098 was undisturbed with lambda-cyhalothrin in the medium, also at the highest doses used. In general, the chemical insecticides gamma-cyhalothrin, lambda-cyhalothrin, and rynaxypyr were the three that least affected the vegetative growth of most of the fungal isolates studied, though gamma-cyhalothrin and lambda-cyhalothrin did produce a significant inhibition of the vegetative growth of LPSc 963 (Table 2).
Results from the generalized linear model on the effects of the chemical insecticides on conidial viability, vegetative growth, and conidia production in the fungal strains
| Parameter | Source | DF | Chi-Square | Pr>ChiSq |
|---|---|---|---|---|
| Conidial viability | Insecticides | 20 | 27.51 | <0.1 |
| Strains | 4 | 173.71 | <0.1 | |
| Insecticides*Strains | 80 | 1.23 | <0.08 | |
| Vegetative growth | Insecticides | 20 | 51.20 | <0.001 |
| Strains | 4 | 84.27 | <0.001 | |
| Insecticides*Strains | 80 | 14.32 | <0.001 | |
| Conidia production | Insecticides | 20 | 284 945 722.60 | <0.001 |
| Strains | 4 | 355 937 902.12 | <0.001 | |
| Insecticides*Strains | 80 | 9 316 535.59 | <0.001 |
Conidial viability, vegetative growth and conidia production of strains Beauveria bassiana LPSc 1067, LPSc 1082, LPSc 1098; Metarhizium anisopliae LPSc 907; and Metarhizium robertsii LPSc 963; both alone and in combination at twice the average concentration of the chemical agents recommended for application in the field (200%), at the average concentration (100%), and at concentrations reduced to 50% and 25% of the average
| Insecticides | Doses (ppm) | Fungal strains* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LPSc 1067 | LPSc 907 | LPSc 1082 | ||||||||
| V | VG | CP | V | VG | CP | V | VG | CP | ||
| Gamma-cyhalothrin | 0 | 98±2.4a | 9±0a | 8.37±43a | 97±2.1a | 9±0a | 8.97±4.23a | 94±2.4a | 9±0a | 1.67±1.73a |
| 13 | 97±1.8a | 9±0a | 87±43a | 97±1.3a | 9±0a | 8.87±4.23a | 94±2.1a | 9±0a | 1.57±1.73a | |
| 26 | 95±2.2bc | 9±0a | 3.17±2.53b | 97±1.9a | 9±0a | 1.47±1.63b | 94±2.5a | 9±0a | 36±72b | |
| 52 | 96±1.4ab | 9±0a | 1.47±1.73c | 95±2.5a | 9±0a | 6.46±1.13c | 92±2.7a | 9±0a | 3.46±82b | |
| 104 | 93±1.2c | 9±0a | 8.36±1.23d | 92±2.2b | 8.2±0.8a | 5.36±13c | 90±3.2b | 9±0a | 3.46±82b | |
| Lambda-cyhalothrin | 0 | 98±2.4a | 9±0a | 8.37±43a | 97±2.1a | 9±0a | 8.97±4.23a | 94±2.4a | 9±0a | 1.67±1.73a |
| 22 | 98±2.4a | 9±0a | 5.17±3.23b | 97±2.8a | 9±0a | 2.17±23b | 91±3.4a | 9±0a | 1.47±1.63a | |
| 44 | 97±1.7a | 9±0a | 1.57±1.73c | 96±1.7a | 9±0a | 6.36±1.13c | 91±2.1a | 9±0a | 9.26±1.33b | |
| 87 | 96±2.3ab | 9±0a | 9.16±1.33d | 94±1.4a | 9±0a | 4.26±9.22c | 92±2.8a | 9±0a | 56±13c | |
| 174 | 92±4.4b | 9±0a | 4.66±9.62e | 89±3.8b | 5.8±1.6b | 3.66±8.52c | 91±2.5a | 9±0a | 3.46±8.23c | |
| Rynaxypyr | 0 | 98±2.4a | 9±0a | 8.37±43a | 97±2.1a | 9±0a | 8.97±4.23a | 94±2.4a | 9±0a | 1.67±1.73a |
| 15 | 97±1.5a | 9±0a | 27±23b | 97±3.6a | 9±0a | 5.96±13b | 93±3.2ab | 9±0a | 8.86±1.33b | |
| 30 | 96±2.2a | 9±0a | 1.57±1.73b | 95±3.4a | 9±0a | 5.66±13b | 92±2.8ab | 9±0a | 8.46±1.23bc | |
| 60 | 96±1.8a | 9±0a | 5.96±13c | 94±2.2ab | 8.8±1.2a | 3.66±8.52b | 90±1.5ab | 8±1.1a | 4.56±92cd | |
| 120 | 92±1.3b | 9±0a | 4.36±9.32c | 90±2.8b | 6.3±0.8b | 3.46±8.32b | 89±2.2b | 6±1.4b | 1.36±5.12d | |
| Luphenuron | 0 | 98±2.4a | 9±0a | 8.37±43a | 97±2.1a | 9±0a | 8.97±4.23a | 94±2.4a | 9±0a | 1.67±1.73a |
| 25 | 95±2.3b | 9±0a | 1.57±1.73b | 93±3.3b | 5.4±1.7b | 6.66±1.13b | 91±2.7ab | 9±0a | 8.46±1.23b | |
| 50 | 93±2.6bc | 7.6±1.1b | 5.26±13c | 93±2.9b | 2.7±1.4c | 4.76±9.72b | 90±3.2b | 8.6±1.7a | 7.96±1.23b | |
| 100 | 90±2.2cd | 4.6±0.8c | 2.86±7.52c | 90±2.3bc | 2.4±0.5cd | 3.96±8.82b | 89±2.3b | 3.1±0.6b | 3.86±8.72c | |
| 200 | 87±1.8d | 1.8±0.6d | 2.46±72c | 87±1.7c | 1.2±0.2d | 3.36±8.12b | 85±2.8c | 1±0.3c | 2.56±7.22c | |
| Methoxyfenocide | 0 | 98±2.4a | 9±0a | 8.37±43a | 97±2.1a | 9±0a | 8.97±4.23a | 94±2.4a | 9±0a | 1.67±1.73a |
| 36 | 95±2.6b | 8.7±2.1a | 1.27±1.53b | 93±3.1b | 6.6±1.9b | 85±42b | 90±2.1b | 9±0a | 6.16±1.13b | |
| 72 | 91±1.5c | 7±1.1b | 4.96±9.92c | 92±3.6b | 5.3±2.1bc | 5.95±3.42b | 90±2.8b | 8.2±2.1a | 4.26±9.22bc | |
| 144 | 89±1.8c | 6.7±0.9b | 2.76±7.42cd | 89±2.8b | 5.1±2.9c | 4.85±3.12b | 88±3.1b | 5.3±2.2b | 2.56±72cd | |
| 288 | 82±2.3d | 6.7±1.2b | 9.66±4.32d | 89±2.2b | 3.1±2.7d | 3.75±2.72b | 88±3.4b | 4.5±1b | 3.35±52d | |
| Insecticides | Doses (ppm) | Fungal strains* | |||||
|---|---|---|---|---|---|---|---|
| LPSc 1098 | LPSc 963 | ||||||
| V | VG | CP | V | VG | CP | ||
| Gamma-cyhalothrin | 0 | 95±3.6a | 9±0a | 1.67±1.83a | 92±3.1a | 9±0a | 2.67±2.23a |
| 13 | 93±3.1ab | 7±2.4b | 1.57±1.73a | 90±3.8a | 3.4±0.8b | 2.57±2.23a | |
| 26 | 93±3.4ab | 7±2.5b | 9.36±1.33b | 92±3.3a | 2.7±0.3b | 1.17±1.53b | |
| 52 | 91±2.9b | 7±2.2b | 8.86±1.33b | 90±3.6a | 2.7±0.6b | 5.76±13c | |
| 104 | 85±3.8c | 6.7±2.3b | 3.86±8.72c | 79±4.6b | 2.2±1.1b | 4.26±9.22c | |
| Lambda-cyhalothrin | 0 | 95±3.6a | 9±0a | 1.67±1.83a | 92±3.1a | 9±0a | 2.67±2.23a |
| 22 | 91±3.8ab | 9±0a | 86±1.23b | 90±2.5ab | 2.4±0.4b | 7.46±1.23b | |
| 44 | 90±3.5bc | 9±0a | 6.96±1.13bc | 87±1.8b | 2.3±0.9b | 4.86±9.82bc | |
| 87 | 86±3.4cd | 9±0a | 4.56±9.52bc | 80±1.1c | 2±0.7b | 4.36±9.32bc | |
| 174 | 83±3.3d | 9±0a | 3.76±8.62c | 72±1.1d | 1.4±0.4b | 2.76±7.32c | |
| Rynaxypyr | 0 | 95±3.6a | 9±0a | 1.67±1.83a | 92±3.1a | 9±0a | 2.67±2.23a |
| 15 | 91±3.3b | 8.8±2.3a | 5.16±13b | 87±3.5b | 7.6±1.8ab | 5.26±13b | |
| 30 | 88±3.2bc | 8.4±2.5a | 4.56±9.52b | 83±3.1b | 7.2±2.4bc | 4.66±9.62bc | |
| 60 | 87.3±3.5bc | 7.6±2.2ab | 4.36±9.32b | 77±2.8c | 6±2c | 3.96±8.82bc | |
| 120 | 85±4.1c | 6.2±2.3b | 2.56±7.22b | 75±3.3c | 4.2±2.2d | 2.16±6.52c | |
| Luphenuron | 0 | 95±3.6a | 9±0a | 1.67±1.83a | 92±3.1a | 9±0a | 2.67±2.23a |
| 25 | 91±4.8ab | 7.5±1.2b | 4.56±9.52b | 83±3.6b | 8.4±1.6ab | 1.86±62b | |
| 50 | 90±5.1bc | 6.9±1.1b | 3.46±8.32b | 77±4.8c | 8.4±1.4ab | 6.95±3.72b | |
| 100 | 87±4.4c | 1.3±0.8c | 5.16±6.82b | 73±4.1cd | 7.1±1.2b | 4.35±2.92b | |
| 200 | 78±4.7d | 1.2±0.1c | 4.56±4.52b | 71±4.5d | 2.8±0.6c | 3.45±2.62b | |
| Methoxyfenocide | 0 | 95±3.6a | 9±0a | 1.67±1.83a | 92±3.1a | 9±0a | 2.67±2.23a |
| 36 | 89±3.7b | 8.4±1.7ab | 4.36±9.22b | 83±3.8b | 9±0a | 8.15±42b | |
| 72 | 87±5.1bc | 7.4±1.1b | 2.56±7.12bc | 75±3.9c | 9±0a | 4.55±32b | |
| 144 | 84±5.5cd | 5.3±1.4c | 1.56±5.52bc | 69±4.7d | 9±0a | 4.35±2.92b | |
| 288 | 80±4.4d | 5.5±0.8c | 3.35±2.52c | 68±4.5d | 8.4±1.1a | 3.15±2.52b | |
CP=a×10bconidia/ml±SD; V=mean percent±SD; VG=cm2±SD.
Mean values followed by the same letters within the same fungal strain are not significantly different according to LSD (p≤0.05). Where large mean values and similarly large standard deviations occur—in the interest of space, we have resorted to using a shorthand notation for expressing those means and the standard deviations as well: Where a base 10 raised to a power would normally be written we have eliminated the base and simply placed the power to which it is raised immediately after the associated number so that what would otherwise, for example, be a×10b is written here merely as ab.
Finally, we found significant differences in the conidia production from all five strains of entomopathogenic fungi when maintained in association with different doses of chemical insecticides (Table 1). The reduction in conidia production was more pronounced with luphenuron and methoxyfenozide at the higher doses of 200ppm and 288ppm, respectively (Table 2). M. robertsii strain LPSC 963 was the most greatly affected by the action of these chemical insecticides, with conidia productions that ranged from 2.6×107±2.2×103conidia/ml in the control to 3.4×105±2.6×102conidia/ml when combined with 200ppm luphenuron and 3.1×105±2.5×102conidia/ml when exposed to 288ppm of methoxyfenozide (Table 2). Insecticides that affected conidia production to a lesser extent even at higher doses were gamma-cyhalothrin and lambda-cyhalothrin. B. bassiana strain LPSC 1067 in particular was the least affected by these latter two insecticides, with conidia-production values ranging from 8.3×107±43conidia/ml in the control to 8.4×106±1.2×103conidia/ml in combination with 104ppm of gamma-cyhalothrin and 4.6×106±9.6×102conidia/ml when exposed to 174ppm of lambda-cyhalothrin (Table 2).
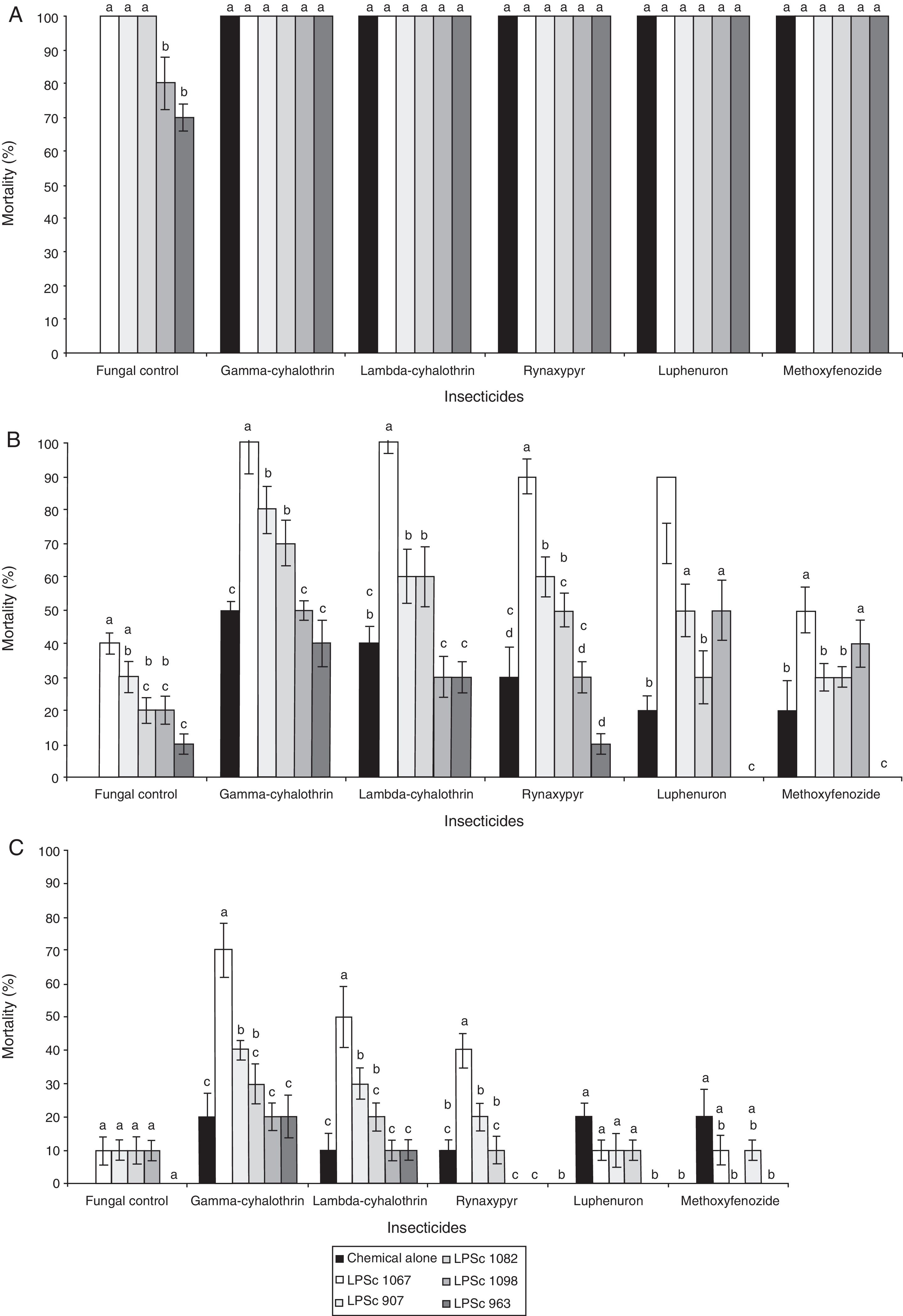
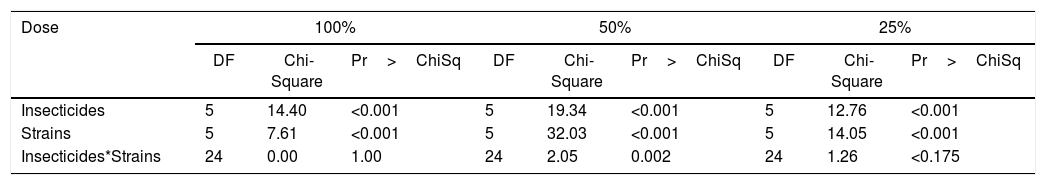
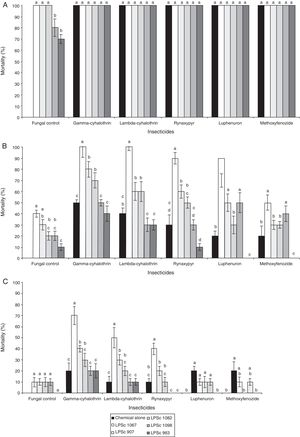
A comparison of the percent mortalities of third-instar larvae of R. nu registered after a 10-day exposure to different combinations of the five insecticides and the five fungal strains revealed significant differences. The interaction insecticides*strains was significant at doses of 50% of the maximum field concentration, but not at 100% or 25% (Table 3). In addition, the percent mortality obtained after exposure of the larvae to the insecticides or to the fungal strains alone was 100%, except for B. bassiana LPSc 1098 and M. robertsii LPSc 963, where the percent mortalities were 80±0.78% and 70±0.67%, respectively (Fig. 1A).
Results of the generalized linear model on the percent mortalities of third instar R. nu larvae after a 10-day exposure at different concentrations of chemical pesticides alone or in combination with fungal strains
| Dose | 100% | 50% | 25% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DF | Chi-Square | Pr>ChiSq | DF | Chi-Square | Pr>ChiSq | DF | Chi-Square | Pr>ChiSq | |
| Insecticides | 5 | 14.40 | <0.001 | 5 | 19.34 | <0.001 | 5 | 12.76 | <0.001 |
| Strains | 5 | 7.61 | <0.001 | 5 | 32.03 | <0.001 | 5 | 14.05 | <0.001 |
| Insecticides*Strains | 24 | 0.00 | 1.00 | 24 | 2.05 | 0.002 | 24 | 1.26 | <0.175 |
Mean percent±SD mortality of Rachiplusia nu exposed to chemical insecticides and entomopathogenic fungi both alone and in combination at (a) the maximum field concentration (100%) and at concentrations of the chemical agents and fungal strains both reduced to (b) 50% and (c) 25%. The bars indicate standard errors, and different letters denote significant differences between fungal strains within each insecticide treatment according to the LSD test (p≤0.05).
Moreover, exposure of R. nu to strains LPSc 1067, LPSc 1082, LPSc 1098, LPSc 907, or LPSc 963 in combination with any one of the insecticides at the maximum field concentrations produced 100% mortality in the larvae in all instances (Fig. 1A). At those maximum field concentrations of both entomopathogenic agents, however, we observed an additive effect with all those combinations (Table 4). At 50% of the maximum concentrations of both the chemical insecticides and the fungal isolates, we also observed a greater percent of mortality of the R. nu larvae after exposure to the two agents in combination than after treatments with those compounds or the fungi alone—except for the combinations lambda-cyhalothrin–LPSc-1098 (30±0.48%), whose lethality was lower than that of the insecticide alone (40±0.23%), or all combinations of the M. robertsii strain LPSc 963, where the percent mortality in combination with any of the chemical insecticides was lower than that caused by those insecticides or the isolate alone (Fig. 1B). In contrast, at this 50% maximum concentration of the two entomopathogenic agents we observed synergism in the combinations gamma-cyhalothrin–LPSc-1067, –LPSc-963, lambda-cyhalothrin–LPSc-1067, rynaxypyr–LPSc-1067, –LPSc-1098, and luphenuron–LPSc-1067 and antagonism with lambda-cyhalothrin–LPSc-1098, –LPSc-963, rynaxypyr–LPSc-963, luphenuron–LPSc-963, and methoxyfenozide–LPSc-907, –LPSc-963 added together; whereas in all other pairings of insecticide and fungus the effect was strictly additive (Table 4). At 25% maximum concentration, the lethality of the insecticides and fungal strains was usually additive in combination—except for the pairings gamma-cyhalothrin–LPSc-1067, lambda-cyhalothrin–LPSc-1067, rynaxypyr–LPSc-1067, and methoxyfenozide–LPSc-1082—where synergism occurred. At this lower concentration, the only combination in which we observed antagonism was with rynaxypyr–LPSc-1098 (Table 4). Finally, the pairing gamma-cyhalothrin–LPSc-1067 produced the highest mortality (70±0.7%). Moreover, at both 50% and 25% of the maximum concentrations of both the insecticides and the fungal isolates, the combinations gamma-cyhalothrin–LPSc-1067 and lambda-cyhalothrin–LPSc-1067 produced the highest percent mortality on the larvae of R. nu (Fig. 1B, C).
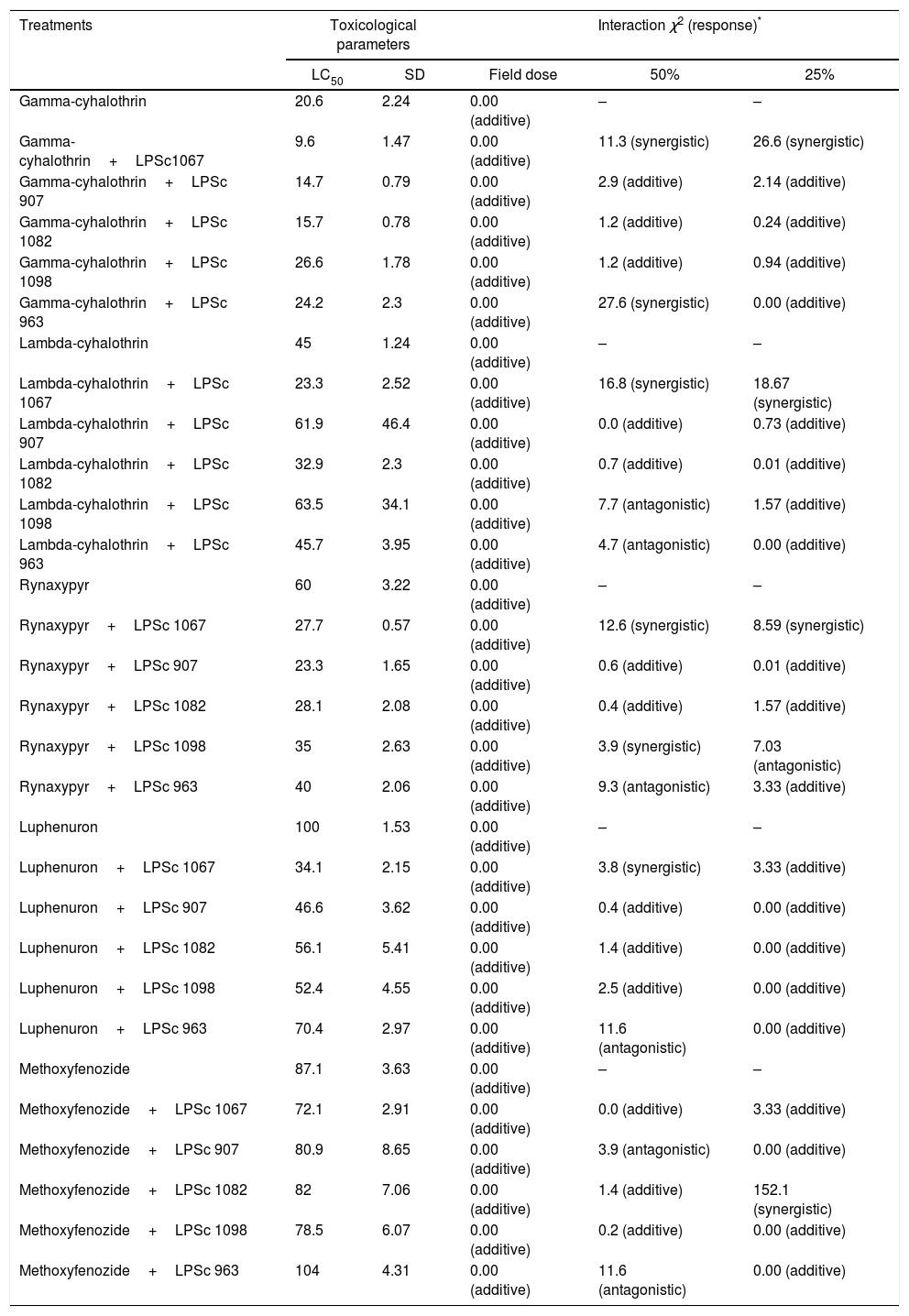
Median lethal concentrations (LC50s) in ppm to Rachiplusia nu of chemical insecticides alone and in combination with the entomopathogenic fungal strains, after a 10-day exposure
| Treatments | Toxicological parameters | Interaction χ2 (response)* | |||
|---|---|---|---|---|---|
| LC50 | SD | Field dose | 50% | 25% | |
| Gamma-cyhalothrin | 20.6 | 2.24 | 0.00 (additive) | – | – |
| Gamma-cyhalothrin+LPSc1067 | 9.6 | 1.47 | 0.00 (additive) | 11.3 (synergistic) | 26.6 (synergistic) |
| Gamma-cyhalothrin+LPSc 907 | 14.7 | 0.79 | 0.00 (additive) | 2.9 (additive) | 2.14 (additive) |
| Gamma-cyhalothrin+LPSc 1082 | 15.7 | 0.78 | 0.00 (additive) | 1.2 (additive) | 0.24 (additive) |
| Gamma-cyhalothrin+LPSc 1098 | 26.6 | 1.78 | 0.00 (additive) | 1.2 (additive) | 0.94 (additive) |
| Gamma-cyhalothrin+LPSc 963 | 24.2 | 2.3 | 0.00 (additive) | 27.6 (synergistic) | 0.00 (additive) |
| Lambda-cyhalothrin | 45 | 1.24 | 0.00 (additive) | – | – |
| Lambda-cyhalothrin+LPSc 1067 | 23.3 | 2.52 | 0.00 (additive) | 16.8 (synergistic) | 18.67 (synergistic) |
| Lambda-cyhalothrin+LPSc 907 | 61.9 | 46.4 | 0.00 (additive) | 0.0 (additive) | 0.73 (additive) |
| Lambda-cyhalothrin+LPSc 1082 | 32.9 | 2.3 | 0.00 (additive) | 0.7 (additive) | 0.01 (additive) |
| Lambda-cyhalothrin+LPSc 1098 | 63.5 | 34.1 | 0.00 (additive) | 7.7 (antagonistic) | 1.57 (additive) |
| Lambda-cyhalothrin+LPSc 963 | 45.7 | 3.95 | 0.00 (additive) | 4.7 (antagonistic) | 0.00 (additive) |
| Rynaxypyr | 60 | 3.22 | 0.00 (additive) | – | – |
| Rynaxypyr+LPSc 1067 | 27.7 | 0.57 | 0.00 (additive) | 12.6 (synergistic) | 8.59 (synergistic) |
| Rynaxypyr+LPSc 907 | 23.3 | 1.65 | 0.00 (additive) | 0.6 (additive) | 0.01 (additive) |
| Rynaxypyr+LPSc 1082 | 28.1 | 2.08 | 0.00 (additive) | 0.4 (additive) | 1.57 (additive) |
| Rynaxypyr+LPSc 1098 | 35 | 2.63 | 0.00 (additive) | 3.9 (synergistic) | 7.03 (antagonistic) |
| Rynaxypyr+LPSc 963 | 40 | 2.06 | 0.00 (additive) | 9.3 (antagonistic) | 3.33 (additive) |
| Luphenuron | 100 | 1.53 | 0.00 (additive) | – | – |
| Luphenuron+LPSc 1067 | 34.1 | 2.15 | 0.00 (additive) | 3.8 (synergistic) | 3.33 (additive) |
| Luphenuron+LPSc 907 | 46.6 | 3.62 | 0.00 (additive) | 0.4 (additive) | 0.00 (additive) |
| Luphenuron+LPSc 1082 | 56.1 | 5.41 | 0.00 (additive) | 1.4 (additive) | 0.00 (additive) |
| Luphenuron+LPSc 1098 | 52.4 | 4.55 | 0.00 (additive) | 2.5 (additive) | 0.00 (additive) |
| Luphenuron+LPSc 963 | 70.4 | 2.97 | 0.00 (additive) | 11.6 (antagonistic) | 0.00 (additive) |
| Methoxyfenozide | 87.1 | 3.63 | 0.00 (additive) | – | – |
| Methoxyfenozide+LPSc 1067 | 72.1 | 2.91 | 0.00 (additive) | 0.0 (additive) | 3.33 (additive) |
| Methoxyfenozide+LPSc 907 | 80.9 | 8.65 | 0.00 (additive) | 3.9 (antagonistic) | 0.00 (additive) |
| Methoxyfenozide+LPSc 1082 | 82 | 7.06 | 0.00 (additive) | 1.4 (additive) | 152.1 (synergistic) |
| Methoxyfenozide+LPSc 1098 | 78.5 | 6.07 | 0.00 (additive) | 0.2 (additive) | 0.00 (additive) |
| Methoxyfenozide+LPSc 963 | 104 | 4.31 | 0.00 (additive) | 11.6 (antagonistic) | 0.00 (additive) |
The number of dead lepidopterans observed and the number of dead lepidopterans expected was used to test the hypothesis of independence with 1 df and p=0.05. Additivity was indicated if χ2<3.84, antagonism if χ2>3.84 and Pc<Pe, where Pc is the mortality observed with the insecticide plus the fungal strain and Pe is the expected mortality of the combination of the two agents. Synergism was indicated if χ2>3.84 and Pc>Pe. SD, standard deviation.
Table 4 lists the toxicologic parameter LC50 determined after a 10-day exposure to each pesticide alone or in combination with one of the fungal strains. Gamma-cyhalothrin alone was the insecticide with the lowest lethal concentration at an LC50 of 20.6ppm, whereas luphenuron manifested the highest at an LC50 of 100ppm. In most instances, a lower LC50 was obtained when combining any of the five pesticides with one of the different fungal isolates, except for the following combinations: gamma-cyhalothrin–LPSc-1098 and gamma-cyhalothrin–LPSc-963, with LC50 values of 26.6 and 24.2ppm, respectively; lambda-cyhalothrin–LPSc-907, lambda-cyhalothrin–LPSc-1098, and lambda-cyhalothrin–LPSc-963, with LC50 values of 61.9, 63.5, and 45.7ppm, respectively, as opposed to 45.0 for the pesticide alone; and methoxyfenozide–LPSc-963 at an LC50 of 104ppm, with the value for methoxyfenozide being 87.1 (Table 4). Furthermore, we found similar values for the LC10 and the NEC sublethal toxicologic parameters: those values were 11.4 and 9.7ppm, respectively, for gamma-cyhalothrin; 40.6 and 39.7ppm for lambda-cyhalothrin; 60 and 60ppm for rynaxypyr; 100 and 100ppm for luphenuron; and 69.8 and 64.31ppm for methoxyfenozide.
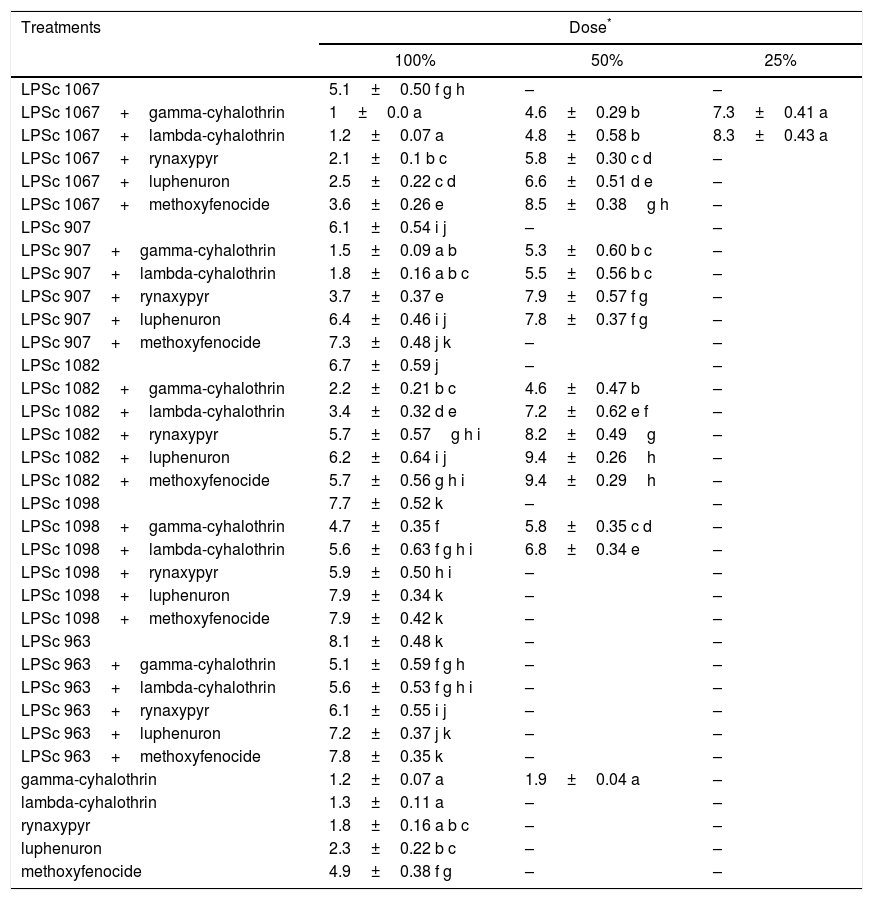
The MST calculated for the strains, the insecticides, and their combinations at 100% concentrations indicated that the most virulent combinations were gamma-cyhalothrin–LPSc-1067 and lambda-cyhalothrin–LPSc-1067 with respective MSTs of 1.0 and 1.2 days, which survival times were significantly different from those recorded after exposure to the rest of the pesticide treatments (Wilcoxon Test, p value <0.05). In contrast, the highest MSTs were observed with the strain LPSc 963 (8.1 days) and with the combinations methoxyfenozide–LPSc-1098 and luphenuron–LPSc-1098 (7.9 days), with those being significantly different from the MSTs recorded with the rest of the treatments (Wilcoxon Test, p value <0.05; Table 5). At a 50% maximum field concentration, the lowest MST was 4.6 days after exposure to gamma-cyhalothrin–LPSc-1067 and gamma-cyhalothrin–LPSc-1082 and 4.8 days in the presence of the combination lambda-cyhalothrin–LPSc-1067, which values were significantly different from those the other pesticide treatments (Wilcoxon Test, p value <0.05; Table 5). At a 25% concentration, the MST could be calculated for only the combinations gamma-cyhalothrin–LPSc-1067 and lambda-cyhalothrin–LPSc-1082, with those survivals accordingly being 7.3 and 8.3 days, respectively (Table 5).
Median survival time in days (±SD) at different concentrations of fungi and pesticides alone or in combination
| Treatments | Dose* | ||
|---|---|---|---|
| 100% | 50% | 25% | |
| LPSc 1067 | 5.1±0.50 f g h | – | – |
| LPSc 1067+gamma-cyhalothrin | 1±0.0 a | 4.6±0.29 b | 7.3±0.41 a |
| LPSc 1067+lambda-cyhalothrin | 1.2±0.07 a | 4.8±0.58 b | 8.3±0.43 a |
| LPSc 1067+rynaxypyr | 2.1±0.1 b c | 5.8±0.30 c d | – |
| LPSc 1067+luphenuron | 2.5±0.22 c d | 6.6±0.51 d e | – |
| LPSc 1067+methoxyfenocide | 3.6±0.26 e | 8.5±0.38g h | – |
| LPSc 907 | 6.1±0.54 i j | – | – |
| LPSc 907+gamma-cyhalothrin | 1.5±0.09 a b | 5.3±0.60 b c | – |
| LPSc 907+lambda-cyhalothrin | 1.8±0.16 a b c | 5.5±0.56 b c | – |
| LPSc 907+rynaxypyr | 3.7±0.37 e | 7.9±0.57 f g | – |
| LPSc 907+luphenuron | 6.4±0.46 i j | 7.8±0.37 f g | – |
| LPSc 907+methoxyfenocide | 7.3±0.48 j k | – | – |
| LPSc 1082 | 6.7±0.59 j | – | – |
| LPSc 1082+gamma-cyhalothrin | 2.2±0.21 b c | 4.6±0.47 b | – |
| LPSc 1082+lambda-cyhalothrin | 3.4±0.32 d e | 7.2±0.62 e f | – |
| LPSc 1082+rynaxypyr | 5.7±0.57g h i | 8.2±0.49g | – |
| LPSc 1082+luphenuron | 6.2±0.64 i j | 9.4±0.26h | – |
| LPSc 1082+methoxyfenocide | 5.7±0.56 g h i | 9.4±0.29h | – |
| LPSc 1098 | 7.7±0.52 k | – | – |
| LPSc 1098+gamma-cyhalothrin | 4.7±0.35 f | 5.8±0.35 c d | – |
| LPSc 1098+lambda-cyhalothrin | 5.6±0.63 f g h i | 6.8±0.34 e | – |
| LPSc 1098+rynaxypyr | 5.9±0.50 h i | – | – |
| LPSc 1098+luphenuron | 7.9±0.34 k | – | – |
| LPSc 1098+methoxyfenocide | 7.9±0.42 k | – | – |
| LPSc 963 | 8.1±0.48 k | – | – |
| LPSc 963+gamma-cyhalothrin | 5.1±0.59 f g h | – | – |
| LPSc 963+lambda-cyhalothrin | 5.6±0.53 f g h i | – | – |
| LPSc 963+rynaxypyr | 6.1±0.55 i j | – | – |
| LPSc 963+luphenuron | 7.2±0.37 j k | – | – |
| LPSc 963+methoxyfenocide | 7.8±0.35 k | – | – |
| gamma-cyhalothrin | 1.2±0.07 a | 1.9±0.04 a | – |
| lambda-cyhalothrin | 1.3±0.11 a | – | – |
| rynaxypyr | 1.8±0.16 a b c | – | – |
| luphenuron | 2.3±0.22 b c | – | – |
| methoxyfenocide | 4.9±0.38 f g | – | – |
A combination of sublethal concentrations of chemical insecticides and entomopathogenic fungi can cause increased stress, immunocompromise, and a consequent alteration in insect physiology and behavior—there especially in survival and reproduction; thus leading to more favorable results in an insect-control program that includes such a biologic component6. A pairing of sublethal concentrations of insecticides with fungal entomopathogens can increase pest mortality as well as reduce the killing time compared to the use of either agent alone10. We observed that although some differences in conidial viability did occur in the presence of the five chemical pesticides investigated—with respect to the vegetative growth and conidia production of all fungal isolates—such differences were either low or insignificant. In many combinations, especially at low insecticide doses, the values were comparable to those of the controls. We observed differences in viability parameters due to toxical effect of the five chemical pesticides investigated in doses-dependent relationship. The vegetative growth of all strains of B. bassiana was not affected by lambda-cyhalothrin and gamma-cyhalothrin; and to a lesser extent by M. anisopliae strain LPSc 907. Also, the conidial production and viability of the five fungal isolates was less affected by gamma-cyhalothrin at concentrations lesser or equals to 50% of recommended dose. In all cases, luphenuron and methoxyfenozide had a greater deleterious effect on viability parameters analyzed. In a similar study Oliveira et al.26 reported that formulations of conventional insecticides exhibit a toxic effect on viability parameters of B. bassiana evaluated in this work. These authors report a great effect on vegetative growth and a variable effect on conidial production being in some cases compatibles with the tested insecticides; however, full recommended doses inhibit the conidial viability.
Although recent reports have indicated that various oil-based surfactants may influence the viability and virulence of fungal entomopathogens in several ways21, nevertheless studies aimed at investigating the in vitro compatibility between insecticides and entomopathogenic fungi have always had the advantage of exposing the fungal pathogen to the maximum action of the chemical compounds, a condition that often does not occur under field conditions. To the contrary, any compatibility indicated in vitro with an insecticide would be strong preliminary evidence in favor of the utility of that combination under field conditions2. Moreover, those latter authors ascertained that conidial germination in the presence of commercial pesticides in vitro was one of the most informative indicators of compatibility for use in the field. In the study reported here, with most of the insecticides at low doses, we observed that the B. bassiana strain LPSc 1067 was the least affected by the action of the five chemical insecticides since that strain obtained a higher percentage of viable conidia with no accompanying change in the vegetative growth and conidia production. In contrast, M. robertsii strain LPSc 963 had the lowest values for the percentage of viable conidia, vegetative growth, and conidia production. Similar results had been observed by Oliveira et al.26, who reported that formulations of insecticides based on triazophos, chlorpyrifos, and endosulfan inhibited the viability of B. bassiana conidia by 100% at the three concentrations used. In this investigation, the five chemical insecticides exhibited varying interactions (i.e., synergism, additivity, or antagonism) with respect to the toxicity against R. nu larvae when combined with the strains B. bassiana LPSc 1067, 1082, 1098; M. anisopliae LPSc 907; or M. robertsii LPSc 963; with mostly additivity being observed. In a total of 10 pairings, however, synergistic responses did occur at either 50% or 25% of the maximum field concentrations of both the chemical insecticides and the fungal isolates, while in 7 combinations we recorded antagonistic effects (Table 4). Based on these observations, certain combinations of the chemical pesticides and entomopathogenic fungi that we tested in this work were particularly effective against R. nu larvae at reduced doses (50% and 25%) because of the occurrence of such synergistic interactions between those agents.
Various authors have reported differing results in the combined action of fungal strains and chemical insecticides in field applications. For example, the combination B. bassiana–Dimilin™ was more effective than a pairing of B. bassiana with other chemical agents in field applications against grasshoppers in the United States10. In populations of grasshoppers in Mali treated with a mixture of B. bassiana and Dimilin™, Delgado et al.8 observed that the decrease in the numbers of insects continued until the end of the monitoring. The so-called insect-growth regulators (IGRs)—i.e., chemical insecticides known to be less detrimental to beneficial insects and more worker-friendly because of an action not involving the insect nervous system—are more environmentally safe than conventional insecticides. For this reason, in recent years, IGRs have been favored for use in integrated-pest-management (IPM) programs32. In the present work, the IGR insecticides luphenuron and methoxyfenozide produced a high mortality at maximum field doses, although at doses reduced to 50% and 25% of those maximum doses lower mortalities were recorded than those documented for the other insecticides. In contrast, the combination gamma-cyhalothrin–LPSc-1067 not only caused the highest percent mortality against R. nu larvae among all the combinations tested but also exhibited a synergistic interaction at 50% and 25% of the maximum field doses. Furthermore, the combinations gamma-cyhalothrin–LPSc-1067 and lambda-cyhalothrin–LPSc-1067 produced a similar percent mortality of R. nu larvae, even at doses of 50% of the maximum.
The pairing of the insecticides gamma-cyhalothrin and lambda-cyhalothrin, in the formulations and doses tested, had no deleterious effects on the percentage of viable conidia, vegetative growth, or conidia production of B. bassiana strain LPSc 1067. In addition, this fungal isolate combined with those pesticides produced the highest percent mortality of R. nu larvae. Therefore, these pesticide-fungus pairings can be recommended for an IPM program aimed at the control of R. nu. However, one of the major challenges for the success of a fungal entomopathogen in the field is the development of an adequate formulation. The fungal formulation process must consider several objectives. Foremost is the stabilization of the organism to allow prolonged storage. In addition, the formulation must be suitable for handling and release in the field, and protect the entomopathogenic fungi from adverse environmental factors.
Future laboratory studies will be needed using B. bassiana LPSC 1067, to the aim of obtaining a stable fungal formulation, before it can be evaluated in combination with the chemical insecticides gamma-cyhalothrin and lambda-cyhalothrin on field trials, for an IPM control of the lepidopteran pests of soybean, R. nu.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest.
This study was partially supported by Consejo Nacional de Investigaciones Científicas y Tecnológicas (PIP 0018), Universidad Nacional de La Plata (UNLP, 11/N 651) and Fondo para la Investigación Científica y Tecnológica (FONCYT; PICT 2013-0543; PICT2015-1146). Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.