To analyze the impact of I/D polymorphism of the angiotensin-converting enzyme gene on the responses of 24 h blood pressure, heart rate variability and nitric oxide after moderate aerobic exercise session.
MethodTwenty seven hypertensive elderly were genotyped for the I/D polymorphism of the angiotensin-converting enzyme gene (D/D: n=9; I/D: n=9; I/I: n=9) and performed a bout of aerobic exercise at 90% of anaerobic threshold. Measurements of mean blood pressure, heart rate variability and nitric oxide were performed before and during 24h post-aerobic exercise session.
ResultsThe D/D genotype showed impaired mean arterial pressure responses (elevation) in the nocturnal sleep (10:30pm to 06:30am) post-aerobic exercise when compared to rest (D/D= −4.2±8.1mmHg; p>0.05, I/D=−9.2±9.1mmHg; p<0.05 and I/I=−7.0±6.9mmHg; p<0.05). Besides, in day time of 06:30am to 10:30am (next day) higher values of mean arterial pressure responses occur between D/D=4.7±4.6mmHg vs. I/D=−2.0±13.0mmHg; p<0.05. The carriers of the D allele, in addition to not release nitric oxide significantly post-aerobic exercise session (p>0.05), showed reduced elevation of nitric oxide between genotypes immediately after aerobic exercise (D/D=12.8±135.3μM and I/D=−8.7±69.0μM vs. I/I=132.9±188.7μM; p<0.05). Reduced elevation of heart rate variability post-aerobic exercise session occurred when compared to rest (D/D=1h: −0.08±0.09s; p<0.05 and 24h: −0.08±0.11s; p<0.05).
ConclusionElderly hypertensive individuals carrying the D/D genotype of the angiotensin converting enzyme gene showed impaired responses of blood pressure post-aerobic exercise, especially during sleep, and reduced heart rate variability during the 24h post-aerobic exercise. Besides, to angiotensin converting enzyme genotypes with the presence of D allele (D/D and I/D) was impaired release of nitric oxide post-aerobic exercise session.
Analizar el impacto del polimorfismo I/D del gen de la enzima conversora de la angiotensina en la presión sanguínea, variación de la frecuencia cardíaca y óxido nítrico durante 24h en respuesta a una batería moderada de ejercicios aeróbicos.
MétodoVeintisiete ancianas hipertensas fueron genotipadas para el polimorfismo I/D del gen de la enzima conversora de la angiotensina (D/D: n=9; I/D: n=9; I/I: n=9) y realizaron una batería de ejercicios aeróbicos al 90% del umbral anaeróbico. Se realizaron medidas de la presión arterial media, variabilidad de la frecuencia cardíaca y óxido nítrico, antes y durante las 24h siguientes después de una sesión de ejercicios aeróbicos.
ResultadosEl genotipo D/D presentó una respuesta inadecuada de la presión arterial media (aumento) durante el sueño nocturno después del ejercicio (22:30-06:30) en comparación con el reposo (D/D=–4.2±8.1mmHg; p>0.05, I/D=–9.2±9.1mmHg; p<0.05 and I/I=–7.0±6.9mmHg; p<0.05). Por otro lado, durante el día, de 06:30 hasta las 10:30 de la mañana (del día siguinete) se registraron valores elevados del aumento de la presión arterial media en el genotipo D/D=4.7±4.6mmHg vs. I/D=-2.0±13.0mmHg; p<0.05. Las portadoras del alelo D, más allá de no mostrar significación en los niveles de óxido nítrico después de la sesión de ejercicio, mostraron una reducción del incremento de óxido nítrico entre los genotipos después del ejercicio aeróbico (D/D=12.8±135.3μM y I/D=-8.7±69.0μM vs. I/I=132.9±188.7μM; p<0.05). Se observó una reducción del aumento de la variabilidad de la frecuencia cardíaca después de los ejercicios, cuando se compara con los valores de reposo (D/D=1h: –0.08±0.09s; p<0.05 y 24h: –0.08±0.11s; p<0.05).
ConclusiónIndividuos ancianos e hipertensos portadores del genotipo D/D del gen de la enzima conversora de la angiotensina mostraron una respuesta inadecuada de la presión sanguínea postejercicio aeróbico, especialmente durante el sueño, y reducción de la variabilidad de la frecuencia cardíaca durante las 24h siguientes tras el ejercicio. Además, el genotipo de la enzima conversora de la angiotensina, con presencia del alelo D (D/D e I/D), tiene la liberación de óxido nítrico alterada, después de la sesión de ejercicio aeróbico.
Analisar o impacto do polimorfismo I/D do gene da enzima conversora da angiotensina na pressão sanguínea, variabilidade da frequência cardíaca e óxido nítrico durante 24h, em resposta a uma bateria moderada de exercícios aeróbicos.
MétodoVinte e sete idosas hipertensas foram genotipadas para o polimorfismo I/D do gene da enzima conversora da angiotensina (D/D: n=9; I/D: n=9; I/I: n=9) e realizaram uma sessão de exercício aeróbio à 90% do limiar anaeróbio. Medidas de pressão arterial média, variabilidade da frequência cardíaca e óxido nítrico foram coletadas antes e durante as 24h seguintes, após a sessão de exercício aeróbio.
ResultadosO genótipo D/D mostrou uma resposta inadequada da pressão arterial média (elevação) durante o sono após o exercício (22:30-06:30) em comparação ao repouso (D/D=–4.2±8.1mmHg; p>0.05, I/D=–9.2±9.1mmHg; p<0.05 e I/I=–7.0±6.9mmHg; p<0.05). Além disso, durante o dia, de 06:30-10:30da manhã (do dia seguinte), maiores valores de pressão arterial média ocorreram entre D/D=4.7±4.6mmHg vs. I/D=-2.0±13.0mmHg; p<0.05. O grupo carreador do alelo D, além de não revelar significância no óxido nítrico após a sessão de exercício, mostrou uma redução da elevação do óxido nítrico entre os genótipos logo após o exercício aeróbio (D/D=12.8±135.3μM e I/D=-8.7±69.0μM vs. I/I=132.9±188.7μM; p<0.05). Ocorreu uma redução do R-Ri após os exercícios quando comparada com os valores do repouso (D/D=1h: –0.08±0.09s; p<0.05 e 24h: –0.08±0.11s; p<0.05).
ConclusãoIndivíduos idosos e hipertensos carreadores do genótipo D/D para o gene da enzima conversora da angiotensina mostraram resposta inadequada de pressão arterial pós-exercício aeróbio, especialmente durante o sono, e reduzida variabilidade da frequência cardíaca durante as 24h seguintes pós-exercício. Além disso, o genótipo da enzima conversora da angiotensina com a presença do alelo D (D/D e I/D) teve sua liberação de óxido nítrico prejudicada nos momentos após a sessão de exercício aeróbio.
Environmental factors1 and genetic characteristics2,3 interact to determine the risk of cardiovascular disease. Among these, hypertension is associated with endothelial dysfunction4 and, in consequence, with a low endothelium dependent vasodilation.5 Furthermore, impaired autonomic modulation with low heart rate variability (HRV) and an increased sympathetic tonus have been associated with high blood pressure.6
Several studies have aimed to investigate the effects of exercise in different groups, showing an important outcome in the acute hemodynamic control by analyzing post-exercise hypotension (PEH). The phenomenon of PEH is well documented7 and, occurs primarily in aerobic8,9 and combined exercise.10 It is important to noteworthy that PEH has a significant and positive correlation with the chronic effects of aerobic training,11 which brings an important clinical application for this phenomenon in the long term.
On the other hand, Hagberg et al.,12 demonstrated that approximately 25% of individuals did not show a decrease in blood pressure (BP) after aerobic training. A body of evidence attributes differential amplitudes of BP attenuation3 and nitric oxide (NO2−) production2 to genetic factors, what prompts for investigating candidate genes to explain such variability.
Polymorphisms of the angiotensin converting enzyme (ACE) gene, located on chromosome 17 in humans, have been found and the polymorphism is characterized by the presence (insertion) or absence (deletion) of a 287-base-pair alu repeat within intron 16 of the gene. The presence of the deletion (D) allele has been associated with higher concentrations of circulating ACE.13,14 The ACE, an element of the renin – angiotensin system, catalyzes the conversion of angiotensin I to angiotensin II, a potent vasopressor, and inactivates bradykinin, a precursor to nitric oxide pathway and a potent vasodilator.14
Some authors15 found no interaction between the I/D polymorphism of the ACE gene with BP responses to exercise in elderly women and others16 showed that allele I of ACE gene elicit a higher post-exercise BP reduction this population. However, none of these studies investigated the BP responses during a 24h post-exercise period, and did not investigate integrated responses of HRV and NO2− which would enable a better view of the event. Thus, the aim of this study was to investigate the impact of I/D polymorphism of the angiotensin-converting enzyme gene on the responses of 24 h blood pressure, heart rate variability and nitric oxide after moderate aerobic exercise session.
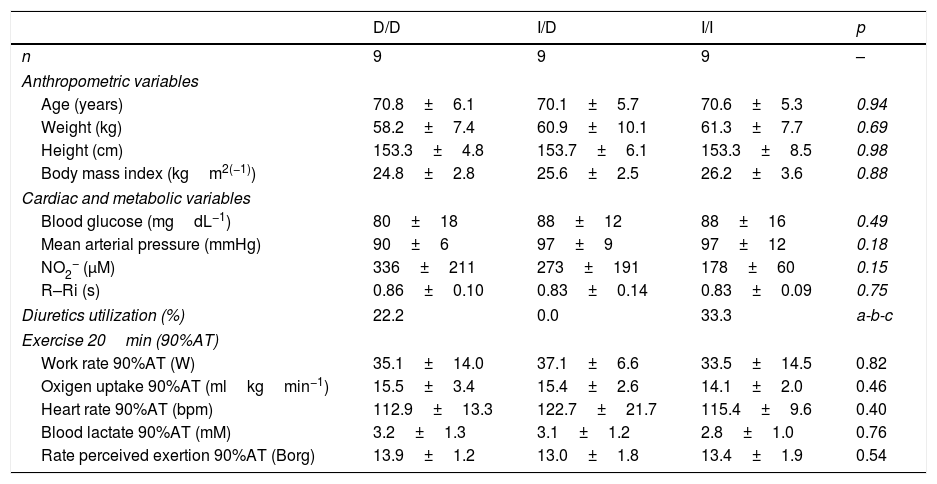
MethodSubjectsElderly women (n=268) with a history of hypertension (V Brazilian Hypertension Guideline) were genotyped for the I/D polymorphism of the ACE gene. The initial sample consisted of volunteers that used or not anti-hypertensive drugs (diuretics, renin-angiotensin system inhibitors, ACE inhibitors, Calcium channel inhibitors, autonomic nervous system inhibitors and beta-blockers). For effective participation in the study, were invited, based on the I/D polymorphism of the ACE gene, 27 elderly women (Table 1), with controlled hypertension due to drug treatment (only diuretics) or changes in lifestyle. All volunteers were informed of the purpose and procedures used in the study and gave their written informed consent to a protocol approved by the institutional review boards of the local university (proc. number 63-2008) and Universal Trial Number (UTN) was U1111-1146-9073.
Baseline characteristics of the sample of hypertensive elderly (n=27).
| D/D | I/D | I/I | p | |
|---|---|---|---|---|
| n | 9 | 9 | 9 | – |
| Anthropometric variables | ||||
| Age (years) | 70.8±6.1 | 70.1±5.7 | 70.6±5.3 | 0.94 |
| Weight (kg) | 58.2±7.4 | 60.9±10.1 | 61.3±7.7 | 0.69 |
| Height (cm) | 153.3±4.8 | 153.7±6.1 | 153.3±8.5 | 0.98 |
| Body mass index (kgm2(−1)) | 24.8±2.8 | 25.6±2.5 | 26.2±3.6 | 0.88 |
| Cardiac and metabolic variables | ||||
| Blood glucose (mgdL−1) | 80±18 | 88±12 | 88±16 | 0.49 |
| Mean arterial pressure (mmHg) | 90±6 | 97±9 | 97±12 | 0.18 |
| NO2− (μM) | 336±211 | 273±191 | 178±60 | 0.15 |
| R–Ri (s) | 0.86±0.10 | 0.83±0.14 | 0.83±0.09 | 0.75 |
| Diuretics utilization (%) | 22.2 | 0.0 | 33.3 | a-b-c |
| Exercise 20min (90%AT) | ||||
| Work rate 90%AT (W) | 35.1±14.0 | 37.1±6.6 | 33.5±14.5 | 0.82 |
| Oxigen uptake 90%AT (mlkgmin−1) | 15.5±3.4 | 15.4±2.6 | 14.1±2.0 | 0.46 |
| Heart rate 90%AT (bpm) | 112.9±13.3 | 122.7±21.7 | 115.4±9.6 | 0.40 |
| Blood lactate 90%AT (mM) | 3.2±1.3 | 3.1±1.2 | 2.8±1.0 | 0.76 |
| Rate perceived exertion 90%AT (Borg) | 13.9±1.2 | 13.0±1.8 | 13.4±1.9 | 0.54 |
D/D, I/D and I/I: genotype of angiotensin-converting enzyme (ACE); NO2−: Nitric oxide concentration; R–Ri: absolute value (s) of mean R–R interval; AT: anaerobic threshold. a: p=0.16 to D/D vs. I/D; b: p=0.61 to D/D vs. I/I; c: p=0.08 to I/D vs. I/I.
In separate days, at the same time of day (09:30am), and with at least a 72h interval between experimental sessions, each volunteer was requested to attend to two sessions: (1) incremental test on a cyclergometer until volitional exhaustion and (2) constant exercise session.
For the constant exercise session, participants performed one exercise session for 20min at an intensity corresponding to 90% of anaerobic threshold (AT) on ciclergometer (Lode Excalibur/Netherlands). To AT determination, the participants were submitted to an incremental test on a ciclergometer (Lode mod. Excalibur/Netherlands) starting at 1min warming-up at 0W, and 15-W increments for every three-minute stage, maintaining cycling at 60rpm until voluntary exhaustion or other interruption criteria were adopted, such as a sudden increase of systolic and diastolic BP to 250/115mmHg, rate of perceived exertion (RPE) at 19–20 or unlevelness of the ST segment in the electrocardiogram or any other abnormal cardiovascular response. Gas samples were collected on a breath by breath basis (Cortex Metalyzer 3B system/Germany) and AT was determined by the analysis of O2 (VE/VO2) and CO2 (VE/VCO2) ventilatory equivalents, were considered to be the intensity correspondent to the moment in which VE/VO2 presented a disproportional augment in relation to VE/VCO2. The protocol used for the incremental test was according to Santana et al.16
All participants were oriented to refrain from the use of caffeine, tobacco, alcohol and physical exercise for at least 48h prior to the experimental sessions. BP, heart rate variability and salivary nitrite concentrations were measured at rest and during the 24h after the experimental sessions.
Total DNA was isolated from peripheral blood according to standard procedures.17 The I/D polymorphism in the human ACE gene was determined by inspection of the electrophoretic profile of polymerase chain-reaction products. Either the 490bp (I allele) or the 190bp (D allele) products were amplified using primers: 5′-CTGCAGACCACTCCCATCCTTTCT-3′ and 5′-GATGTGGCCATCACATTCGTCAGAT-3′, which flank the polymorphic site. Inspection of DD subjects was carried out using oligonucleotides (5′- TGGGACCACAGCGCCCGCCACTAC-3′ and 5′-TCGCCAGCCCTCCCATGCCCATAA-3′) specific to amplify a 335bp fragment of the insertion sequence. All procedures to DNA analysis were performed as described by Moraes et al.17
The aerobic exercise session were preceded by a pre-session stage consisting of seating for 20min in a comfortable chair placed in a quiet room. The HRV, measured by R–R interval (R–Ri) and heart rate (HR) (Polar®S810i/Polar Electro/Finland) were measured18 full time resting and mean arterial pressure (MAP) was measured at each 5min of the pre-exercise resting. At each 15min throughout 1h of post-exercise session, recovery the R–Ri and MAP were measured with the participant under conditions similar to the pre-exercise stage. In addition, the measurements of MAP were carried out after the exercise session every 15min throughout the 23h following of daily life activities of the participants (Cardios Dyna MAPA/Brazil). The R–Ri was also measured on 24h post-aerobic exercise session.
In the end of 20min of pre-session resting at seated position as well as in the immediately after exercise session (RAE) the NO2− was measured. At each 15min throughout 1h of post-exercise recovery the NO2− was measured following the same procedures as for pre-exercise measure. Besides, the NO2− was also measured on 24h post-exercise session. To analyze the NO2− the saliva was collected with a cotton swab (Salivette/Sarstedt®) which was chewed for one minute. Then it was centrifuged according to the manufacture instructions and stored in −20°C for latter analysis. Dosage of NO2− a nitric oxide metabolite was done through the Griess’ colorimetric method.19,20
Statistical analysisAn exploratory analysis (Shapiro–Wilk test) was used to verify data normality and then descriptive statistics were performed. Data are presented as means (±SD and ±SE). In addition, the delta variation (absolute variation from pre-exercise to post-exercise values) were calculated for comparison. Two-Way ANOVA (Time [pre and post-session] * Genotype [D/D, I/D and I/I]) was used. Post hoc LSD test for multiple comparisons was adopted to identify possible differences. In addition, MAP values were grouped by average in moments, being “Daytime” for the period from 10:30am to 10:30pm, “Sleep” for the period from 10:30pm to 06:30am and “Daytime” of the next day for the period from 06:30am to 10:30am. For the R–Ri the moments were “Post-exercise (1h)” with an average of 1h post-exercise and “Post-exercise (24h)” for the time of 24h post-exercise session. For NO2− the moments were “Post-exercise (RAE)” to immediately after exercise, “Post-exercise (1h)” to the average of 1h post-exercise and “Post-exercise (24h)” for 24h post-exercise session. The level of significance was set at p<0.05 (Statistica®version 6.0).
ResultsThe general characteristics and functional results among genotypes groups during aerobic exercise session were similar (Table 1).
Regarding the valid measures of ambulatory BP across genotypic groups, it was possible to observe that the measures were 88% to I/I genotype group, 90% to I/D genotype group and 94% to D/D genotype group.
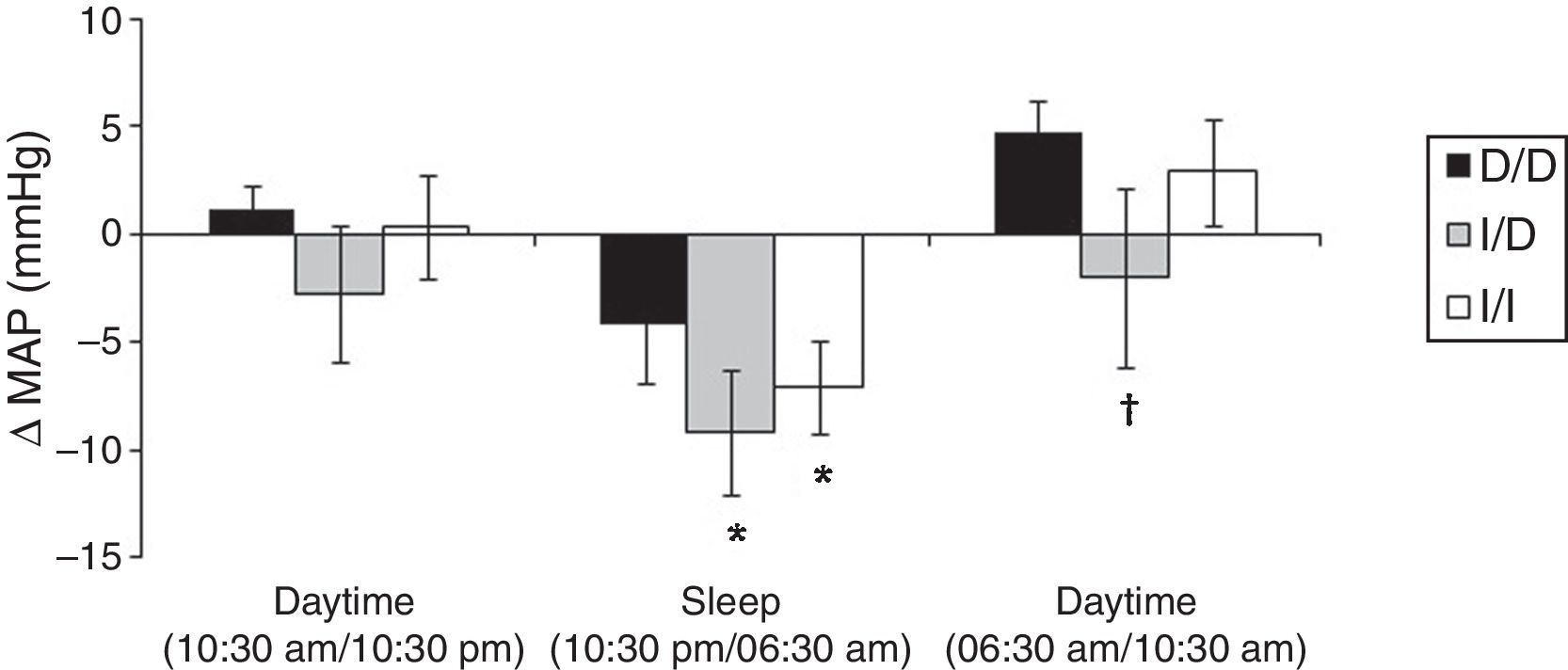
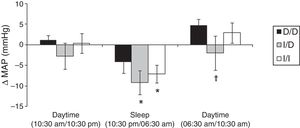
Fig. 1 show the mean variation of absolute values obtained in the ΔMAP to DD vs. I/D vs. I/I genotypes, considering the values of recovery post-exercise minus pre-exercise session. The I/I and I/D genotype groups presented decrease on ΔMAP during sleep (p<0.05). In daytime of 06:30am to 10:30am (next day) higher values of ΔMAP occur to D/D group vs. I/D group (p<0.05).
Furthermore, protective effect on MAP for groups with the presence of allele I occurred when compared to D/D group at 4h post-exercise (I/I=−0.5±2.2 and I/D=−2.9±3.4 vs. D/D=3.4±1.7mmHg; p<0.05) and 20h post-exercise (I/I=−8.2±2.6 and I/D=−11.4±2.6 vs. D/D=−3.2±2.7mmHg; p<0.05).
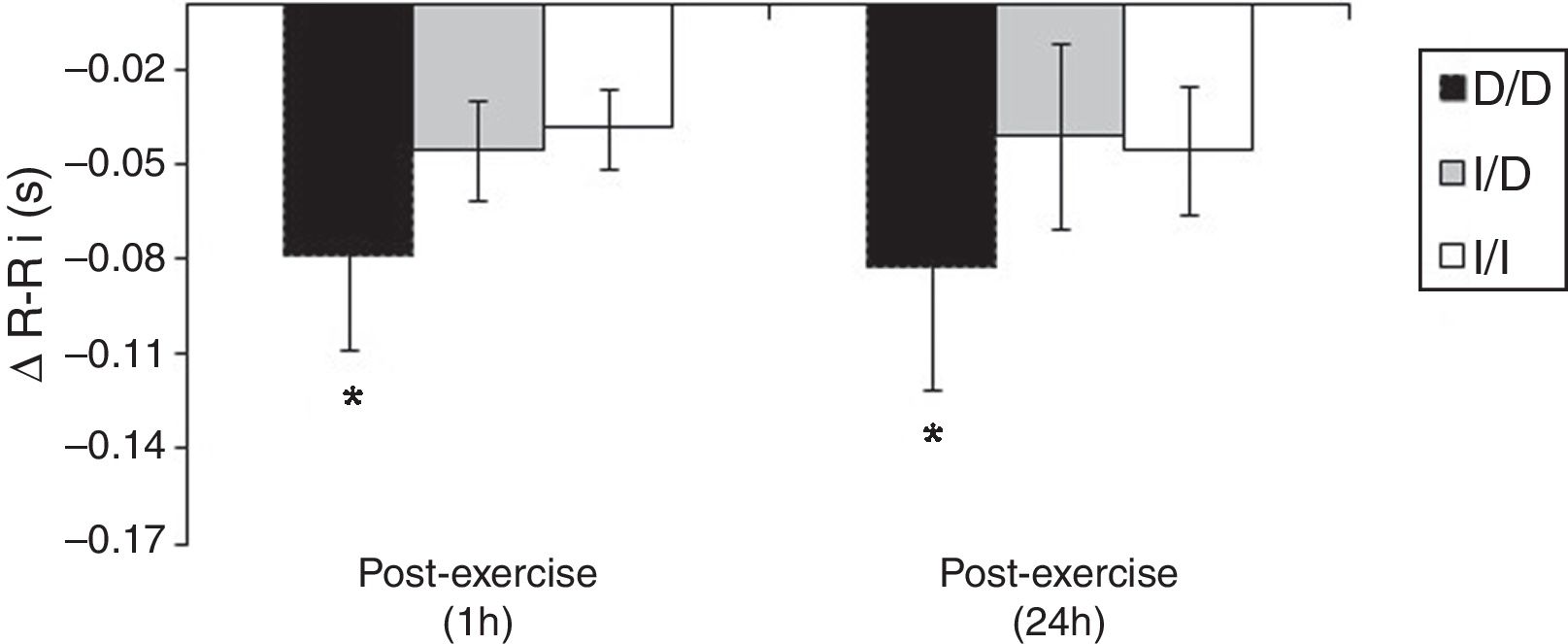
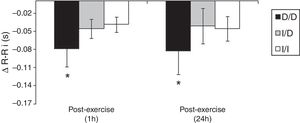
The Fig. 2 shows that, unlike the I/D and I/I genotype groups, considering the values of recovery post-exercise minus pre-exercise session, D/D genotype group decreased the R–Ri at mean 1h and 24h post-exercise (p<0.05).
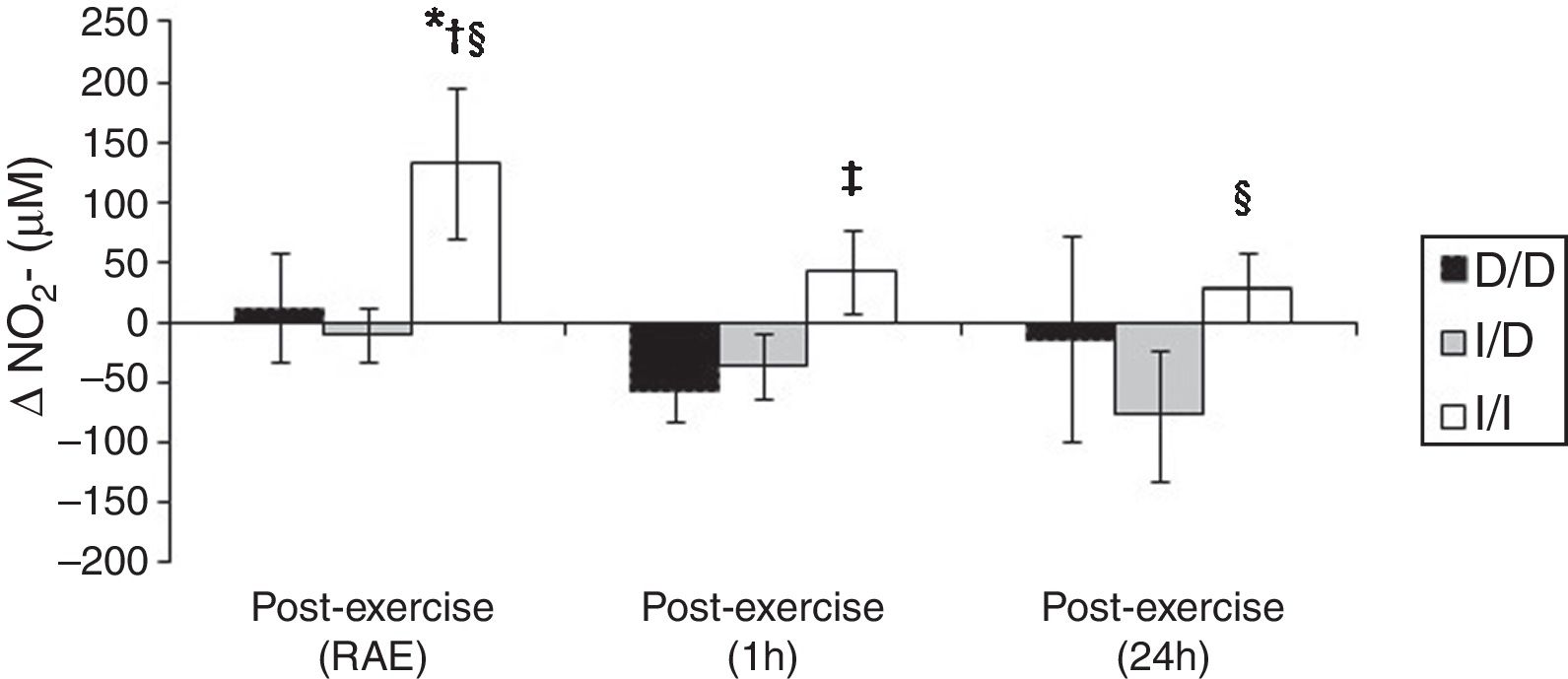
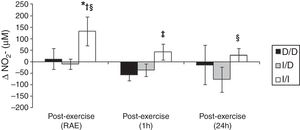
The Figure 3 shows that I/I genotype group increased the NO2− in the RAE moment (p<0.05). Besides, in RAE moment the I/I group presented protective effect with increase of NO2− release when compared to D/D and I/D genotype groups (p<0.05). A similar effect occurred on the mean 1h and 24h post-exercise when compared to D/D group (p=0.06) and I/D group (p<0.05), respectively.
DiscussionOur main findings revealed that the ACE D/D genotype seems to show impaired responses of BP to a moderate aerobic exercise session, especially during sleep in elderly hypertensive individuals, and to ACE genotypes with D allele (D/D and I/D) impaired release of NO2− in moments post-aerobic exercise session. In addition, the D/D genotype showed low HRV (R–Ri) during the 24h after the moderate exercise session. Until the present moment, no study had analyzed, simultaneously, the BP responses during 24h, HRV and NO2− according to a 20min session of aerobic exercise at intensity of 90%AT, in elderly women with a history of hypertension, divided by the I/D polymorphism of the ACE gene and with similar characteristics (Table 1).
One possible explanation for the effects of the I allele and mainly of the I/I genotype of the ACE gene after aerobic exercise on MAP (Fig. 1), can be related to the analyses of both the R–Ri (Fig. 2) and the NO2− (Fig. 3). Unlike the D/D genotype group, the I/I and I/D genotype groups showed a lower reduction of R–Ri during the mean period of 1h and 24h after aerobic exercise when compared to pre-exercise session, which may be reflected in a better control of the MAP. Furthermore, only the I/I genotype group showed a significant release of NO2− when compared to the D/D and I/D genotype groups in moments of the 24h post-exercise. This condition could impair a possible endothelium-dependent vasodilatation induced of aerobic exercise in D/D and I/D groups. These results can be, in part, associated to the nocturnal decrease of MAP that occurred after exercise in the I/I genotype group (Fig. 1). The I/D group, although not show significant release of NO2− post-exercise, also showed a nocturnal decrease in MAP after exercise, possibly because they also presented a lower reduction of R–Ri, in accordance to the I/I group (Fig. 2).
Due to the impaired responses of BP during sleep found in the D/D genotype carriers, it is noteworthy the nocturnal dip of BP. Jones et al.,21 suggest that the BP should reduce during sleep more than 10% compared to previous daytime. In the present study we verified that, during sleep, the variation of nocturnal dip to MAP was to D/D genotype between –15/+11%, I/D genotype between −15/+2% and I/I genotype between −20/+4%. These results show more variation, but particularly positive values for D/D genotype group, which may reflect a greater cardiovascular risk factor,21 even after performing moderate aerobic exercise. As a clinical application, these results suggest that for D/D genotype carriers other types, intensities or duration of physical exercise should be investigated in order to enhance the nocturnal dip in BP. On the other hand, for individuals with the presence of the I allele (I/D and I/I), which showed significant reduction in MAP during sleep (Fig. 1), it is important to remember that acute reduction may correlate positively with chronic effects of aerobic training.11
Although the phenomenon of PEH is well documented,7 this study did not show a significant occurrence of PEH in daytime in any investigated genotypes (Fig. 1). The inexistence of PEH during total daytime may be related do the moderate intensity that was performed (below AT), a low volume of exercise (20min) and specificity of the sample (elderly hypertensive).
Some authors15 found no interaction between the I/D polymorphism of the ACE gene with BP responses to exercise in elderly women and others16 showed that allele I of ACE gene elicit a higher post-exercise BP reduction in this population. Once methodological differences occur, comparisons with other studies are difficult to be carried out, especially with regard to the time of measurement of MAP, where other studies15,16 BP measurements were performed only for 1h post-exercise recovery.
The NO2− is considered a predictor of the activity of endothelial nitric oxide synthase19 and thus a possible marker of endothelium-dependent vasodilatation, phenomenon that could be impaired in the carriers of the D allele during and post-aerobic exercise session (Fig. 3). This condition could not be happening only because of arterial aging,22 since the I/I group is equivalent in age to the I/D and the D/D groups (Table 1) and showed increase in NO2− post-aerobic exercise session. The lower release of NO2− in carriers of the D allele, especially during a high demand of the cardiovascular system (aerobic exercise session), can contribute to the explanation of a possible endothelial dysfunction and vascular disease in these groups,23 event now genotype-dependent. Damages in endothelium-dependent vasodilatation could be preceded by a lower bioavailability of endothelial nitric oxide in individuals with higher activity of the circulating ACE (D/D genotype), since this enzyme allows the degradation of bradikinin,14 which is associated to nitric oxide release by increase in activity of endothelial nitric oxide synthase19 and consequently PEH responses.9
The significant decrease of the R–Ri in the D/D group in the 1h mean and 24h post-aerobic exercise session (Fig. 2) could be associated with a higher activity of the sympathetic tone during the 24h post-exercise recovery period. Ashley et al.,24 investigating the autonomic balance of athletes in a multi-sport competition, found that the low frequency component, which reflects the sympathetic tone's activity, was augmented in the participants of the D/D group when compared to the others after the competition. The explanation of a higher sympathetic nervous activity in the carriers of the D/D genotype seems to be related to the various events of the renin-angiotensin system (RAS). The D/D genotype expresses higher levels of the ACE in hypertensive individuals.13 The ACE plays an important role in the conversion of angiotensin I to angiotensin II, a powerful vasoconstrictor.14 Clemson et al.,25 found that healthy youngsters showed higher plasmatic norepinephrine after the infusion of intra-brachial angiotensin II. The main limitation of this study was the lack of measurement of ACE activity in the different genotype groups. However, the literature13,14 has shown that ACE activity in genotype D/D has almost twice as high when compared with genotype I/I. Jalil et al.,13 demonstrated that hypertensive individuals between 45 and 60 years showed different levels of circulating ACE between extreme homozygotes (I/I=18.0±4.0 vs. D/D=31.0±8.0U/l; p<0.01).
The integrated response of BP, HRV and NO2− 24h after aerobic exercise session between the different genotypes of the ACE gene can be bring thoughts about exercise prescription to individuals of the same age and sex, but with different genetic traits. This topic has to be better investigated in hypertensive elderly, in way to analyze the different forms and best dose of physical exercise to this population. Besides, these results open a possibility of early detect, during and after to a single bout of aerobic exercise, impaired cardiovascular responses that can be related to genetic characteristics of individuals.
We concluded that elderly hypertensive individuals carrying the D/D genotype of the ACE gene seem to have impaired BP responses post-aerobic exercise session, especially during sleep, and to ACE genotypes with presence of D allele (D/D and I/D), impaired release of NO2− in moments post-aerobic exercise session. In addition, the D/D genotype showed low HRV (R–Ri) during the 24h after the moderate aerobic exercise session.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.