To determine possible changes in serum concentrations of pro- and anti-inflammatory cytokines of eutrophic rats subjected to aerobic or resistance physical training.
MethodsThis study examined serum concentrations of TNF-α, IFN-γ, IL-6, IL-10 and IL-1-β in rats that performed aerobic or resistance training for 16 weeks. Thirty-five Wistar rats (male adult) were divided into three groups: Control Group (CG), Aerobic Group (AG) and Resistance Group (RG). Rats were sacrificed 48h after the final training session. Serum concentrations of cytokines were analysed by ELISA.
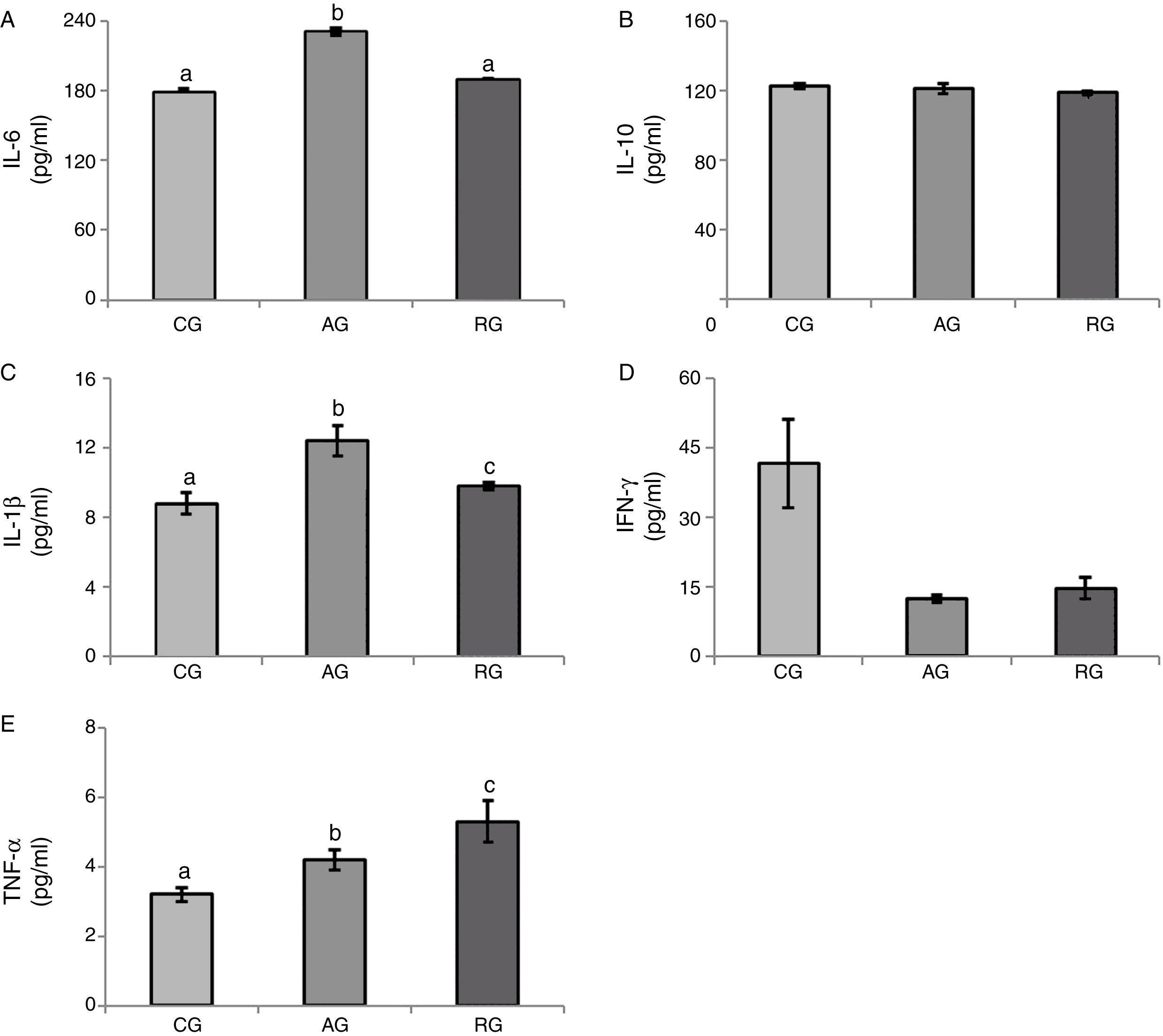
ResultsTNF-α levels were higher in the RG, followed by the AG and CG groups (p<0.001). IFN-γ and IL-10 levels were not significantly different between groups (p=0.097 and p=0.17, respectively). The levels of IL6 and IL1-β were higher in AG compared to RG and CG (p=0.0004 and p=0.003, respectively). In general, our results indicate a higher pro-inflammatory profile in AG and probably RG animals.
ConclusionFurther studies are required in order to better clarify the effects of aerobic and resistance exercise training on pro- and anti-inflammatory cytokines. Additionally, future studies should address the metabolic and molecular pathways involved in these responses.
Determinar posibles cambios en las concentraciones séricas de citoquinas pro y antiinflamatorias de ratas eutróficas sometidas a entrenamiento físico aérobico y de resistencia.
MétodoSe examinaron las concentraciones séricas de TNF-α, IFN-γ, IL-6, IL-10 e IL-1-β en ratas sometidas a entrenamiento aeróbico o de resistencia de 16 semanas de duración. Treinta y cinco ratas Wistar (macho adulto) fueron divididas en 3 grupos: Grupo Control (GC), Grupo Aeróbico (GA) y Grupo Resistencia (GR). Las ratas se sacrificaron 48 horas después de la sesión de entrenamiento final. Las concentraciones séricas de las citoquinas se analizaron por ELISA.
ResultadosLos niveles de TNF-α fueron mayores en el GR, seguido por los grupos de GA y GC (p<0.001). Los niveles de IFN-γ e IL-10 no fueron significativamente diferentes entre los grupos (p=0.097 y p=0.17, respectivamente). Los niveles de IL-6 y IL-1-β fueron mayores en GA comparado con GR y GC (p=0.0004 y p=0.003, respectivamente). En general, nuestros resultados indican mayor perfil antiinflamatorio en GA y probablemente en GR.
ConclusiónSe necesitan estudios adicionales para aclarar mejor los efectos de un entrenamiento aeróbico o de resistencia en las citoquinas pro y antiinflamatorias. Además, los estudios futuros deben abordar las vías metabólicas y moleculares involucradas en estas respuestas.
Determinar as possíveis alterações nas concentrações séricas de citocinas pró e anti-inflamatórias de ratos eutróficos submetidos a treinamento físico aeróbio ou resistência.
MétodoEste estudo examinou a concentração sérica de TNF-α, IFN-γ, IL-6, IL-10 e IL-1-β em ratos que realizaram treinamento aeróbio e de resistência durante 16 semanas. Trinta e cinco ratos Wistar (macho adulto) foram divididos em 3 grupos: grupo controle (GC), grupo aeróbico (GA) e grupo de resistência (GR). Os ratos foram sacrificados 48 horas após a sessão de treino final. As concentrações séricas de citocinas foram analisadas por ELISA.
ResultadosOs níveis de TNF-a foram maiores no GR, seguindo-se os grupos GA e GC (p<0.001). IFN-γ e os níveis de IL-10 não foram significativamente diferentes entre os grupos (p=0.097 e p = 0.17, respectivamente). Os níveis de IL-6 e IL-1-β foram maiores em GA, quando comparados com GC e GR (p=0.0004 e p=0.003, respectivamente). Em geral, os nossos resultados indicam um perfil pró-inflamatório maior nos animais do GA e, provavelmente, no GR.
ConclusãoMais estudos são necessários para melhor esclarecer os efeitos do treinamento físico aeróbico e resistência sobre as citocinas pró e anti-inflamatórias. Além disso, estudos futuros devem abordar as vias metabólicas e moleculares envolvidas nestas respostas.
Noncommunicable chronic diseases (NCCDs) are currently responsible for the majority of deaths in many countries. The main NCCDs related to these deaths are cardiovascular disease, cancer, chronic respiratory diseases, diabetes mellitus and mental illness. It is known that the emergence of NCCDs is related to exposure to risk factors such as hypertension, smoking, hypercholesterolemia, low consumption of fruits and vegetables, overweight and obesity, alcohol abuse and physical inactivity1.
In relation to a sedentary lifestyle, physical activity levels have declined worldwide, with sedentary behaviour at work and during leisure time, as well as the use of “passive” means of transport partly explaining this phenomenon. Physical inactivity is responsible for causing 6% of all deaths and is the fourth major global mortality risk factor. Several authors have demonstrated an association between physical inactivity and NCCDs.1 Moreover, the association between NCCDs and its risk factors (metabolic syndrome, type 2 diabetes mellitus, obesity and atherosclerosis, for example) and systemic low-grade inflammation in different populations has been investigated and established.2,3
Obesity is a clear example of this association since the increase in visceral fat induces increased secretion of pro-inflammatory cytokines, and results in the initiation of low-grade chronic inflammation.4 Cytokines can be classified according to their function, namely pro-inflammatory (interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), tumour necrosis factor alpha (TNF-α), interferon (IFN), among others) or anti-inflammatory (interleukin-4 (IL-4), interleukin-10 (IL-10), interleukin-13 (IL-13), and receptor antagonist IL-1 (IL-1ra)), characterized by inducing the increase in the inflammatory process or its decrease, respectively. Thus, chronic low-grade inflammation is due, among other factors, to the increased production of pro-inflammatory cytokines.5,6
Studies have shown the protective effect of regular exercise against inflammation. This may be attributed, at least in part, to the anti-inflammatory response generated by exercise, which is partly mediated by IL-6 derived from skeletal muscle. Physiological concentrations of IL-6 stimulate the appearance of anti-inflammatory cytokines in the circulation and inhibit the production of pro-inflammatory cytokines.7
In addition, IL-6 appears to stimulate lipolysis and fat oxidation. The anti-inflammatory effects of regular exercise may protect against insulin resistance induced by TNF-α. It has also been proposed that IL-6 and other cytokines, which are produced and released by skeletal muscles (i.e., IL-15), exert their effects on other organs. Thus, they may be termed myokines,8 which may mediate the beneficial effects of physical exercise as they play an important role in protecting the body against diseases associated with systemic low-grade inflammation. On the other hand, when the IL-6 is not produced by the skeletal muscle, but by the adipose tissue (in this case, IL-6 it would become an adipokine), it exerts pro-inflammatory effects.4,9
However, important issues regarding the performance of physical exercise still need to be elucidated, that is: what is the best exercise (aerobic or resistance) for the production of anti-inflammatory cytokines at the expense of pro-inflammatory that, in turn, will act positively on healthy bodies, via the blood stream, on other tissues? Another area that needs further investigation is the ability of exercise-induced skeletal muscle contraction to self-regulate both the pro-inflammatory mechanisms and the anti-inflammatory processes.
Thus, the present study was conducted in order to determine possible changes in serum concentrations of pro- and anti-inflammatory cytokines of eutrophic rats subjected to aerobic or resistance physical training. The initial hypothesis of the study is that there will be positive changes in the concentration of cytokines in animals subjected to aerobic or resistance training compared to a sedentary group. The relevance of this work consists in the possibility of providing theoretical and practical evidence in relation to mechanisms of anti- and pro-inflammatory cytokines originating from two distinct modes of physical training.
MethodAnimalsAll procedures followed specific Brazilian resolutions with respect to bioethics in animal experiments (Law no. 6638, of May 8, 1979 and Decree no. 26645 of July 10, 1934). This study was approved by the Ethics Committee for Research on Animals of the Federal University of Mato Grosso, Cuiabá, Brazil (Protocol 23108.4916/12-0).
Thirty-five male Wistar rats (Rattus norvegicus), that were 60 days old at the start of the experiment were used for the investigation. The Central Animal House of the Federal University Mato Grosso provided the animals. Animals were placed in collective cages (six animals per cage) made of polyethylene and kept in a room where the temperature was maintained at 22±2°C, 50–55% humidity, and controlled under a 12h light–dark cycle (lights on at 0600h and off at 1800h). Animals were provided with commercial rodent feed (Presence®) and water ad libitum.
The animals were randomly divided into three groups:
- •
Control Group (n=12; CG): rats kept sedentary throughout the experimental period.
- •
Aerobic Group (n=11; AG): rats subjected to physical training of swimming, 90–205 days of age.
- •
Resistance Group (n=12; RG): rats subjected to resistance physical training (jumps into the water), of 90–205 days of age.
Body weight as well as food and water intake were measured once a week (Sartorius®, precision of 10g).
Experimental proceduresLiquid environment acclimationWater acclimation was carried out according to the protocol of Voltarelli et al.10 This acclimation was performed with the purpose of reducing stress in the animals during physical exercise, yet without promoting physiological adaptations arising from chronic exercise training. This involved maintaining animals belonging to trained groups in contact with deep water at a temperature of 31±1°C (thermometer, Initial®), for 2 weeks, 5 days a week for 60min.11
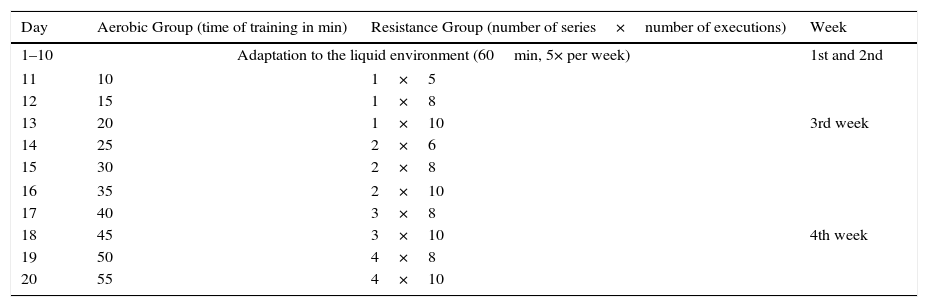
Adaptation period to chronic physical trainingIn the two consecutive weeks following acclimation to water, the animals were subjected to a period of adaptation to physical training with lead overloads (small bags of fabric [cotton] with elastic and Velcro®) tied to their chests. The group that performed aerobic swim training carried a load equivalent to 5% of the body weight.10 The group subjected to resistance training, did so by jumping into the water with a burden equivalent to 50% of their body weight.12 The physical training schedule was performed according to the description contained in Table 1.
Schedule of adaptation to the liquid environment and the physical training of swimming and resistance.
| Day | Aerobic Group (time of training in min) | Resistance Group (number of series×number of executions) | Week |
|---|---|---|---|
| 1–10 | Adaptation to the liquid environment (60min, 5× per week) | 1st and 2nd | |
| 11 | 10 | 1×5 | 3rd week |
| 12 | 15 | 1×8 | |
| 13 | 20 | 1×10 | |
| 14 | 25 | 2×6 | |
| 15 | 30 | 2×8 | |
| 16 | 35 | 2×10 | 4th week |
| 17 | 40 | 3×8 | |
| 18 | 45 | 3×10 | |
| 19 | 50 | 4×8 | |
| 20 | 55 | 4×10 | |
Aerobic Group ran the training during the stipulated time for each day of adaptation. From day 14, the Resistance Group had its exercise divided into series. Between each series, rats were removed from the water and remain at rest in a cage for 1min.
At the end of the adaptation period, the rats were able to perform the physical training by themselves without any prompting from investigators.
Physical trainingRats belonging to both trained groups performed the physical training in a pool, with partitions so that the animals were able to perform the exercises without contact with each other. This was done so that the mechanics of the movement of the two types of physical training were performed correctly.
The rats belonging to AG carried out overload equivalent to 5% of the body weight (small bags [cotton] with elastic and Velcro®, tied to the chest), 1h, five times per week, during 16 weeks. The depth of water was appropriate to rats do not touch the tail in the bottom of the swimming pool.10,11
On the other hand, the animals belonging to RG carried out overload equivalent to 50% of the body weight (cloth bags [cotton] Elastic and Velcro®, tied to the thorax), five times per week, during 16 weeks. The resistance physical training consisted of jumps in water at a depth of 150% in relation to the naso-anal length of the animal; animals performed four series of jumps (10 jumps in each bout); the interval between each series was 1min.12
Both physical training protocols are considered to be of moderate intensity.10–12
Collection of biological samplesRats belonging to all groups were sacrificed 48h after the last training session by CO2 inhalation followed by cervical dislocation. Blood samples were collected (5mL) for determination of inflammation biomarkers.
Biomarkers of inflammationThe serum concentrations of IL1-β, TNF-α, IFN-γ, IL-6 and IL-10 were determined. Protocols for determination were similar because they have been standardized (commercial kits). Blood samples were collected, centrifuged for 15min (Centrifuge Centribio 80-2B, Curitiba, PR, Brazil), for collection of 1.5mL serum. Serum was collected in Eppendorf tubes and stored at −80°C in a biofreezer for further analysis. The concentrations of IL-1β, IL-10 and IL-6 were determined by Boster Immunoleader® Fremont kits (CA, USA). TNF-α levels by Thermo Scientific® kit, Inc. (MA, USA) and IFN-γ concentration by Kit Biolegend®, Inc. (MA, USA). The concentrations were analysed using enzyme immunoassay (ELISA) and the absorbance determined on a microplate reader (Keuha, MN, USA).
Statistical analysisData were analysed using SPSS 20.0 (SPSS Inc., Chicago, Illinois, USA) and were expressed as mean±standard error. In order to verify the normality, we used the Kolmogorov–Smirnov test. To compare the mean, we used the Kruskal–Wallis test and the post hoc Dunn analysis, when necessary. The significance level was pre-set at p<0.05.
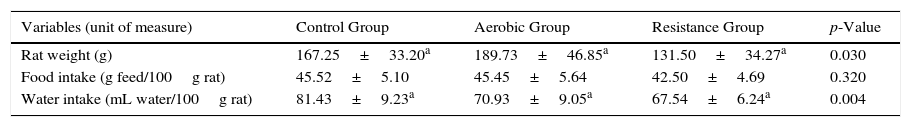
ResultsVariables related to health of the animals are presented in Table 2.
Variables related to health of the animals.
With respect to IL-6 levels, significantly higher values were observed (p<0.001) in AG (231.2±3.2) when compared to that observed in RG (188.9±2.0) and CG (178.2±3.2) groups.
Serum levels of IL-10 were statistically similar (p=0.17) between all groups (AG: 121.2±3.2; RG: 118.7±0.8; CG: 122.4±1.5).
With regards to the serum concentrations of IL-1β (pg/mL) significantly higher values were found (p=0.003) in AG (12.4±0.9) when compared to RG (9.8±0.2) and CG (8.8±0.6), with RG>CG.
Serum levels of IFN-γ (pg/mL) did not differ significantly (p=0.097) in all three experimental groups (AG: 12.4±0.9; RG: 14.6±2.3; CG: 41.6±9.5).
Serum concentrations of TNF-α (pg/mL) were significantly higher (p=0.009) in the RG (5.3±0.6) when compared with AG (4.2±0.3) and CG (3.2±0.2), with AG>CG (Fig. 1).
Serum concentrations of interleukin-6 (IL-6) (A); interleukin-10 (IL-10) (B); interleukin-1beta (IL-1β) (C); interferon gamma (IFN-γ) (D); and tumour necrosis factor-alpha (TNF-α) (E). Results are expressed as mean±standard error. Kruskal–Wallis test with Dunn's post hoc, significance level p<0.05. CG: Control Group; AG: Aerobic Group; RG: Resistance Group. Statistical differences marked by letters (a≠b≠c).
In general, our results indicate a higher pro-inflammatory profile in AG and probably RG animals.
DiscussionIt is known that exercise induces inflammation, which occurs to promote the repair and remodelling of damaged tissue, whereas many cells and components developed during the inflammatory process interact, in a local and systemic manner, to assist in this process. The primary aim of this mechanism is the re-establishment of the organic homeostasis after a single session (acute exercise) or several exercise sessions (chronic effect from exercise training).7 The present study examined the effect of aerobic and resistance training on the serum concentrations of pro- and anti-inflammatory cytokines as well as the body weight, and food and water intakes of animals. As general, by observing the food intake of animals, we noticed that there was no statistical difference between the groups, that is, this suggests that physical training did not change the hunger or satiety of animals. However, we noted statistical difference in water intake, which was higher in the CG; this result may be due, at least in part, to the water intake during physical training performance, since they were performed in liquid environment all the time. However, it was not possible to measure this intake, becoming impossible the deep explanation of this finding. We also observed a decrease in the weight gain of trained animals, which was more pronounced in RG.
IL-6 was higher in the group subjected to the aerobic exercise. It is known that this type of activity, which involves greater participation of different muscle groups, causes a systemic increase in the concentration of this cytokine.13 On the other hand, Lira et al.6 did not observe the same response after training on a treadmill. These authors, on analysing the extensor digitorum longus muscle, found decreased protein expression of IL-6, whereas in the soleus muscle the same was not true. These findings are in opposition to the results of the present study. Donatto et al.14 analysed the expression of IL-6 protein in muscle from healthy mice and with induced tumour subjected to training on vertical overhead ladder and overload with 75–90% of body weight, found that mice with tumours presented IL-6 levels 90% higher than the control rats.
Nunes et al.15 found a decrease in IL-6 serum levels in rats with chronic heart failure that underwent physical training on a treadmill for 8 weeks. As mentioned earlier, in our study, IL-6 levels were higher in the group trained by swimming compared to other groups (AG and RG), showing that the type of ergometer and the organism studied (eutrophic or pathological) may generate different responses for each cytokine. Begue et al.16 analysed the expression of IL-6/STAT-responsive gene and phosphorylation of STAT3 in the deep flexor digitorum after acute resistance exercise and after 10 weeks of physical training; they found that after both an acute session and after chronic physical training, IL-6 mRNA remained upregulated, thereby justifying the hypertrophy after 10 weeks of training, since IL-6 is capable of activating satellite cells.17 This study partly confirms the results of our investigation regarding the justification of the IL-6 increase in AG. Interestingly, the same was not observed in the RG group animals. Perhaps, the training volume (1h for the AG group and four sets of 10 jumps to the RG) may have affected these results.
The mean levels of IL-10 of the three groups analysed in this study were statistically similar. Nunes et al.,15 in a study with rats with chronic heart failure (CHF) and subjected to physical training on a treadmill, found that the serum IL-10 was higher if compared to the sedentary animals, also affected by the CHF. In another study, Nunes et al.18 observed that IL-10 presented by CHF sedentary rats were lower when compared to CHF trained rats and normal animals (sedentary and trained), showing that physical training may cause alterations in this anti-inflammatory cytokine, regardless of the presence of CHF or not. Indeed, this was not observed in our study, and an explanation for this is that the training time that we used (16 weeks) may have contributed to this result, since most studies used protocols of 8–12 weeks of physical training.
Lira et al.19 compared groups of rats submitted to different intensities of exercise (trained and induced overtrained) in four sessions of daily training and with or without recovery time between sessions (recovered and overtraining). These authors observed that the serum levels of IL-10 were higher in the group to which recovery was allowed, as this manoeuvre favoured the increase of an anti-inflammatory cytokine, which hypothetically prevented the occurrence of overtraining phenomenon in animals. These findings correlate with the results of the present study, because the animals belonging to the trained groups showed no signs of overtraining. We emphasize also, that the period of rest of the animals was always on weekends (Saturday and Sunday). However, we believe that this period of rest was not enough for elevation in the levels of IL-10 in these animals, suggesting a reduction in the days of physical training (we used 5, and perhaps should have used 3) in future studies.
Serum levels of IL-1β were higher in AG compared to others, with RG>CG. It is known that IL-1β acts in conjunction with TNF-α in the production of substances that facilitate leucocyte adhesion. We suggest that higher levels of IL-1β in AG are due, at least in part and in a hypothetical way, to the need for increased recruitment of muscles involved in the execution of the exercise, because AG exercised for the duration of 1h continuously, while RG performed four series of jumps, ending the activities soon after. This fact may have led to an increase in the concentration of this cytokine, which is necessary for the synthesis of acute phase proteins by the liver, mainly acting on the containment of systemic inflammation.
Two important studies have been conducted on animals and IL-1β. Recently, Pervaiz and Hoffman-Goetz20 observed in mice subjected to acute aerobic exercise (treadmill), increase in IL-1β concentration in intestinal lymphocytes. Previously, Mussi et al.,21 in a study with rats affected by ischaemia and difficulties in reperfusion, found that after physical training on a treadmill for 8 weeks, elevated serum levels of IL-1β, or both after acute session such as the end of a 10-week physical training period, the levels (serum and/or organic) of IL-1β were increased, confirming the results of our study, especially with regard to the AG group. There are no studies in the literature that determine IL-1β after resistance training in rats, being our study the first one; this fact undermines the comparison of our findings and possible speculation. On the other hand, it is important to emphasize that animals belonging to RG presented values of this cytokine higher than the CG. In addition, it is noteworthy that RG was the group with higher levels of TNF-α, which may have affected IL-1β elevation in this group.
IFN-γ levels were statistically similar in the three groups. Lesniewski et al.22 determined the serum concentration of IFN-γ in elderly rats that exercised voluntarily (activity wheel). The authors observed that this cytokine was lower in the exercised animals, which do not agree with our results. Similarly, and although it was done with humans (there are no studies with animals vs. aerobic exercise and/or IFN-γ vs. resistance exercise), the work done by Golzari et al.,23 verified that serum levels of IFN-γ were lower in women with multiple sclerosis after 8 weeks of combined training (aerobic+resistance). Farinha et al.,24 analysed pro- and anti-inflammatory cytokines in women with metabolic syndrome before and after 12 weeks of moderate aerobic training, which promoted inflammatory process improvement, i.e., increased levels of anti-inflammatory cytokines and decreased concentrations of pro-inflammatory, including IFN-γ.
In this sense, the sedentarism condition resembles the pathological condition with regard to IFN-γ, showing that regular exercise seems to be a great ally to reduce this cytokine. IFN-γ is considered one of the most important pro-inflammatory cytokines, because it acts directly on the response to intracellular bacteria. Therefore, based on our finds, we suggest that both types of physical training were not able to improve the functioning of immune system of the animals, once it was not observed significant differences in relation to this variable in all groups analysed.
In this study, RG had higher serum levels of TNF-α, with AG>CG. These results are controversial with respect to literature, since previous studies have shown a decrease in TNF-α, the main pro-inflammatory cytokine. Pervaiz and Hoffman-Goetz20 observed in mice subjected to acute aerobic exercise, a decrease in TNF-α concentration in intestinal lymphocytes. Our results do not support the above finding. It is known that higher levels (not exacerbated) of TNF-α can be related to the suppression of lipogenesis via the inhibition of the enzyme lipoprotein lipase.25
A relevant question inherent to our study is that physical training protocols (both aerobic and resistance) were applied for 16 weeks (the vast majority of studies use 8–12 weeks of training). We suggest that many of the responses related to cytokines are associated to this important issue, since the total time of physical training can influence these variables in different ways. Unfortunately, we did not perform the determinations on all variables in the eighth week of physical training for comparison purposes. This is another limitation of the present study that will be addressed in future studies in our laboratory.
Future research regarding the best type of exercise in relation to the pro- and anti-inflammatory cytokines are required, since there are no studies in the literature with this characteristic, with the present investigation being the pioneering work.
Finally, studies that address the metabolic and molecular pathways involved in these responses need to be conducted to improve understanding of the question of aerobic training vs. resistance physical training with respect to the overall cytokine profile; it is important to point out that the training time used in this study (16 weeks) may have influenced some results, for example, the increase in TNF-α, IL-1β and IL-6 in both groups when compared to the sedentary animals.
Conflict of interestThe authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was funded by grant from the Brazilian Foundation “National Council for Scientific and Technological Development (CNPq)” (Process number: 470071/2011-7). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.