Introduction
Human limbal epithelial cells (hLEC) are stem cells located at the intermediate zone between the cor-neal crown and the scleral brim.1 hLEC give rise to the corneal epithelium, and limbal cells are also responsible for corneal epithelial tissue repair and complete regeneration after injury.2
The limbal stem cell profile is defined as p63, ABCG2, cytokeratin (CK) 19, and vimentin positive cells, confirming their origin from the basal limbal epithelium, and lack of CK3, CK12, connexin 43 and connexin 50, usually expressed in other epithelial cells at the ocular surface.3
When a pathogen contacts the ocular surface (corneal, conjunctival and limbal epithelial cells) activates the innate immune system through Toll-like receptors (TLR), and induces a complex cascade of events, increasing expression of inflammatory cytokines, such as IL-8 and IL-6.4,5 These cytokines are involved in inflammation and repair of ocular surface; nevertheless both, inflammation and repair, could be deleterious to the eye and may lead to corneal vascularization and corneal opacity.5,6
Ophthalmic infectious diseases (viral7-10 and fungal keratitis11) have been treated with human dialyzable leukocyte extracts (hDLE). Clinical observations in hDLE-treated patients, have suggested an apparent control of ocular inflammatory injuries without changes in the re-epithelialization process.
Dialyzable leukocyte extracts are constituted by numerous peptide sequences below 12KDa, and includes several peptides named transfer factors (TF), ranging between 1.0 and 6.0 kDa.12 These TF are able to transfer specific immune response from healthy donors to healthy receptors,13 however specific TF production is very expensive and instead of them hDLE are currently used in the clinical practice. In this context, hDLE have been widely used as adjuvant for treating patients with infectious diseases and/or with deficient cell-mediated immune response.11,14,15 hDLE are able to induce the expression of mRNA and IFN-γ secretion in peripheral blood mononuclear cells from humans9,11,16 and in animal models17,18(reviewed in 15); however their function related to the apparent control of inflammation at the ocular microenvironment is unknown.
Objective
To determine the inflammatory cytokine profile in supernatants of human limbal epithelial cells after culture with human dialyzable leukocyte extracts.
Methods
Reagents. Bovine fetal serum and keratinocyte serum free medium were purchased from Gibco (Grand Island, NY, USA); Dispase II was purchased from Roche (Mannheim, Germany); mouse anti human cytokeratin antibody was purchased from DakoCytomation (Carpinteria, CA, USA); mouse monoclonal antibodies directed against human cytokeratin 19, anti-p63, and fluorescein isothiocyanate (FITC-) labelled an- ti-vimentin, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); amphotericin B, gentamicin, tripan blue, tripsin/EDTA, saponin, sodium azide and salts to prepare buffers were purchased from Sigma (St. Louis Missouri, USA); bovine serum albumin was purchased from Calbiochem (La Jolla, CA, USA); goat anti mouse IgG1 antibody FITC-labelled was purchased from Southern Biotech (Birmingham, AL, USA); goat anti mouse IgG2a antibody phycoerythrin (PE)-labelled was purchased from US biological (Swampscott, MA, USA)
Human Dialyzable Leukocyte Extracts. hDLE (Transferon®) were kindly donated by the Transfer Factor Project, Escuela Nacional de Ciencias Biologicas, Instituto Politécnico Nacional. (Mexico City, MX)
Isolation and Culture of Human Limbal Epithelial Cells. Sclerocorneal rings from human cadaveric donors were used to obtain limbal cells (Banco de Ojos - Fundación Conde de Valenciana, Mexico). Briefly, the limbal rim was cut into pieces of about 2 mm2 x 2 mm2, each piece was put on a well inside a culture plate (Costar, Corning, NY, USA). All explants were then cultured in supplemented keratinocyte serum-free medium (KSFM) at 37°C, 5% CO2, and 95% humidity, according to Luna-Baca.19 When epithelial cells were observed at the bottom, tissue fragments were removed from well. Cell culture was followed until cell confluence. Purity evaluation of cultured cells was performed with immunofluorescence assays. After purity assessment was done, 2x105 hLEC were cultured with supplemented-KSFM and hDLE (0.5μg/mL or 5 μg/mL) or with Phorbol-Myristate-Acetate-Ionomycin (PMA-Ion) (5 ng/mL - 0.2 μg/mL, respectively). Cell cultures were ended at different times (1h, 3h, 6h and 24h) and supernatants (SN) were collected and stored at -20°C until analysis for soluble cytokines with cytometric bead arrays.
Phase contrast microscopy. To evaluate cell morphology, phase contrast microscopy was performed with confluent cultured cells using an inverted microscope (Olympus CK2) (Melville, NY, US). Cells were documented with a digital camera (Pixe LINK PL-A642) (Otawa, ON, CAN), and pictures were acquired and analysed with Image Pro Plus software v.5.1 (Bethesda, MD, US)
Immunofluorescence assays. To evaluate purity of cultured cells, immunofluorescence was performed according to previously reported methods.19 Briefly, harvested cells were washed once with phosphate-buffered saline (PBS), fixed in 4% p-formaldehyde for 10 min, and then washed and blocked with 10% bovine serum albumin - 0.1% sodium azide in PBS for 15 min. Then, cells were permeabilized with saponin buffer (0.1% saponin, 10% bovine serum albumin, 1% sodium azide in PBS) 10 min; after that, cells were incubated with the first-step mAb for 30 min at room temperature (anti-cytokeratin or anti-cytokeratin 19 or anti-p63). After incubation, cells were washed twice with saponin buffer and a second-step staining was performed in dark, with FITC- or PE- labelled monoclonal antibodies at room temperature (goat IgG against mouse IgG1 FITC-, goat anti-mouse Ig-G2a PE- or FITC-labelled anti-vimentin). Incubation was ended at 30 min, then cells were washed twice with PBS, fixed with 1% p-formaldehyde and analysed in a flow cytometer.
Flow cytometric analysis. 5000 events were acquired by duplicate on a FACScan flow cyto-meter and analysed with CELLQUEST software v. 5.2.1. (Becton Dickinson, Franklin Lakes, NJ, US) To analyse the staining of markers, the acquired cells were gated by their physical properties (forward and side scatter). Data are presented in histograms. Control stains were performed using isotype-matched mAb of unrelated specificity FITC- or PE-labelled.
Determination of soluble cytokines. IL-1β, IL-6, IL-8, IL-12p70, and TNF-α (Human Inflammation Cytokine Kit, BD Biosciences, CA, USA) were measured in SN of culture, with cytometric bead arrays (CBA), following manufacturer's instructions (BD Biosciences). Results were analyzed by flow cytometry with BD CBA software v. 1.1.1. Kit detection limits were as follows: IL-1β, 7.2 pg/mL; IL-6, 2.5 pg/mL; IL-8, 3.6 pg/mL; IL-12p70, 1.9 pg/ mL; and TNF-α, 3.7 pg/mL.
Ethics. The Tenets of the Declaration of Helsinki were followed to process human tissues. This study was approved by the Scientific and Ethics Committees at the Instituto de Oftalmología Fundación Conde de Valenciana, Mexico City.
Statistical Analysis. Kruskal Wallis ANOVA test was used to detect significant differences between groups, and a p <0.05 was considered as statistically significant.
Results
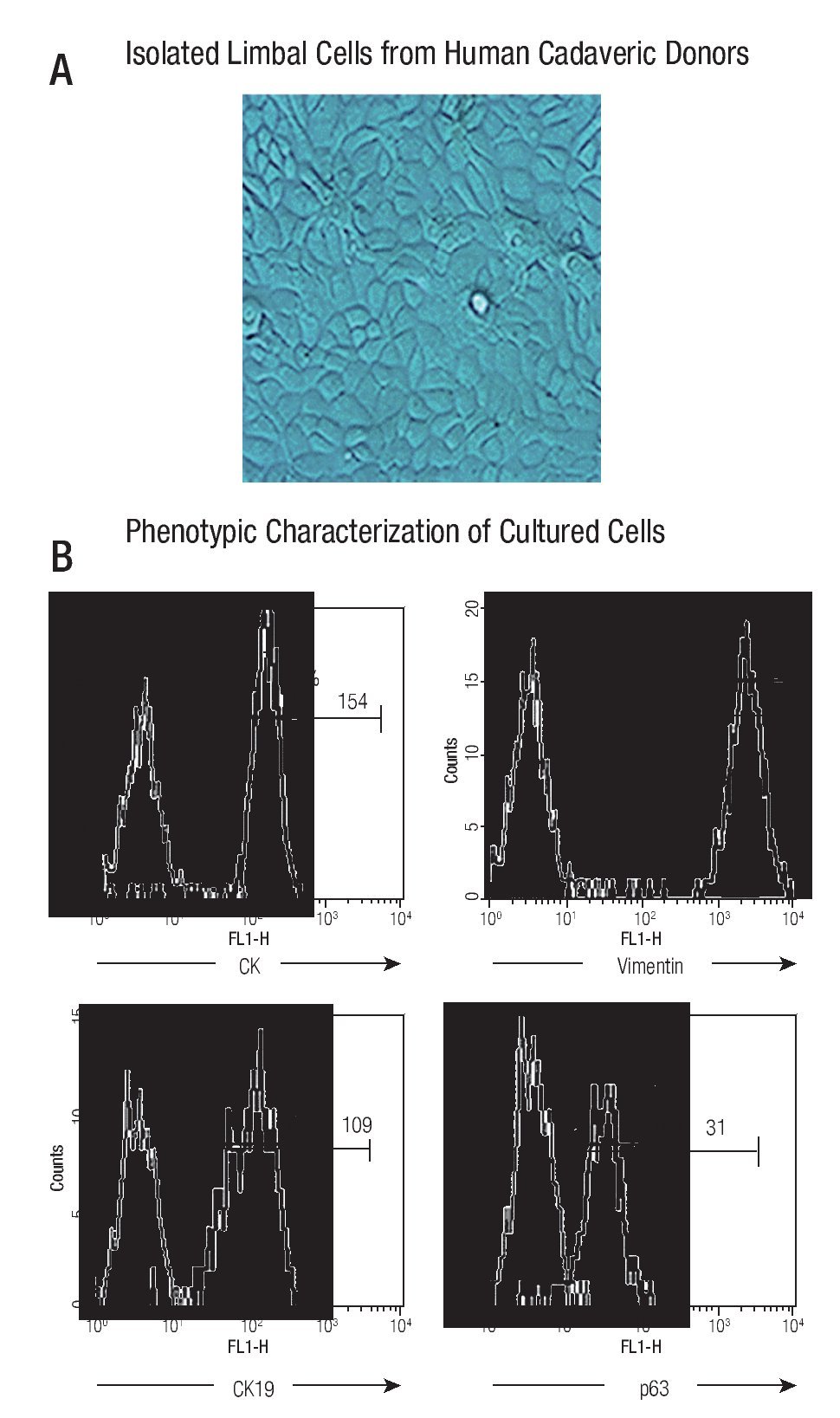
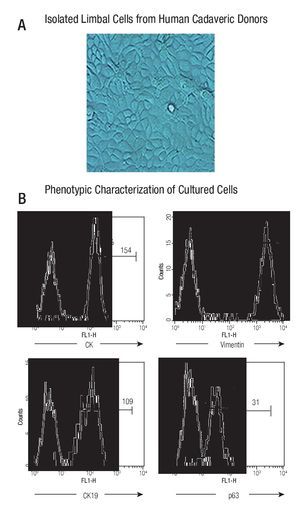
The cultured cells preserved their stem-phenotypic characteristics. Cultured cells reached confluence (90% - 95%) at approximately 20 days of isolation. Cellular morphology was examined with phase-contrast microscopy; ~90% of cultured cells had typical epithelial aspect (Figure 1A). Phenotypic characterization showed that majority of them were cytokeratin (CK) + (98%, with a Mean Fluorescence Intensity, MFI = 154), vimentin + (99%, MFI = 2130), CK19+ (97%, MFI = 109), and p63+ (92%, MFI = 31) (Figure 1B), resembling a limbal stem cell phenotype.
◊ Figure 1. Phenotypic characterization of human limbal epithelial cells. (A) Phase contrast microscopy showing the typical epithelial aspect of isolated limbal cells from human cadaveric donors (400x); (B) Representative histograms showing percentage and mean fluorescence intensity (MFI) of epithelial marker (CK) and stem cell epithelial associated markers (CK19, vimentin, p63) (Thick line). Thin line denotes isotype controls.
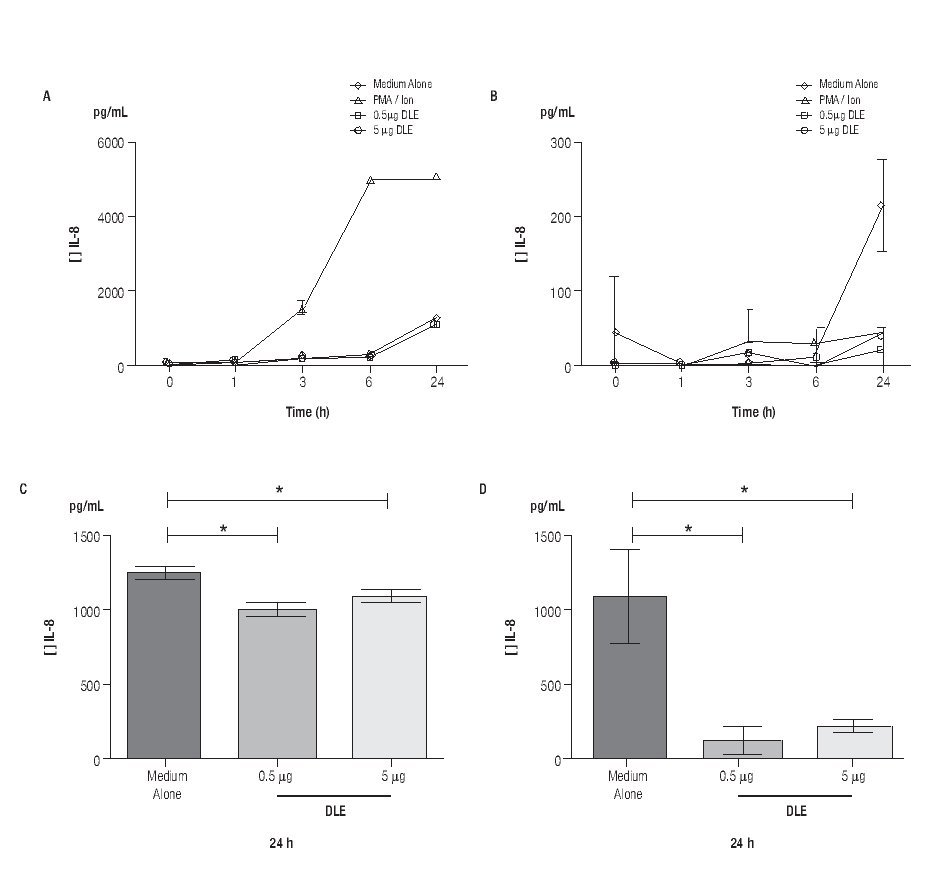
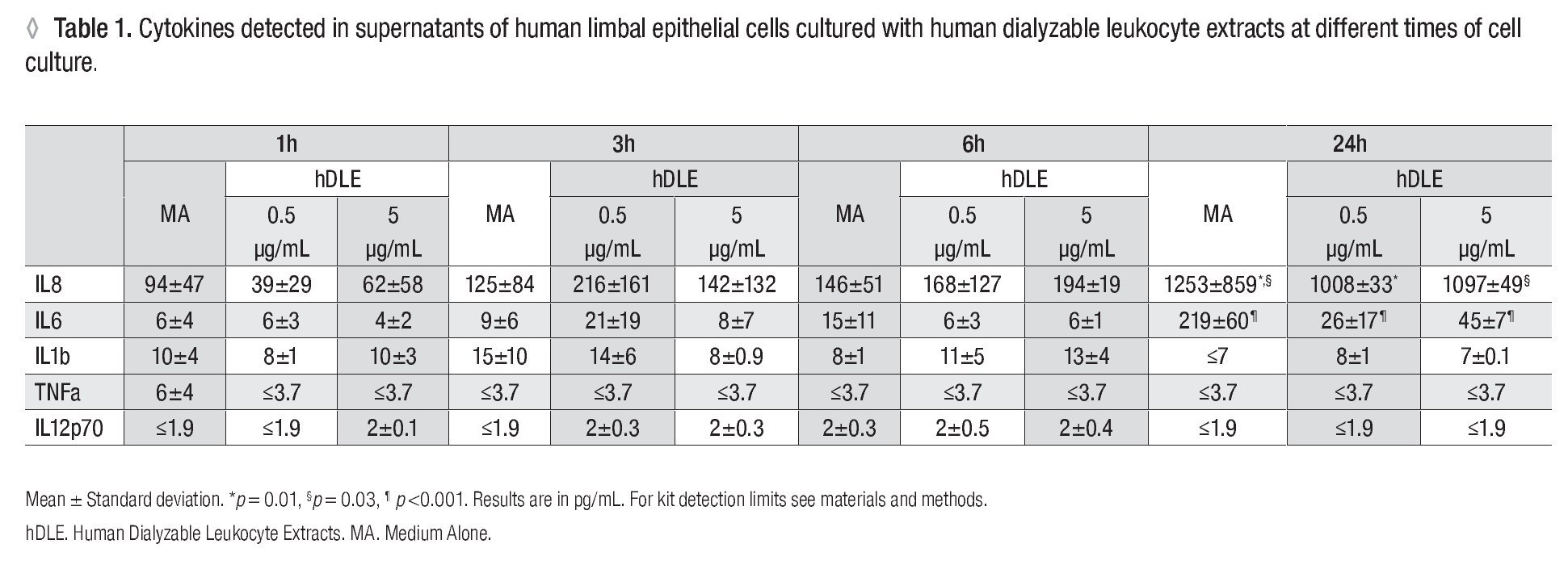
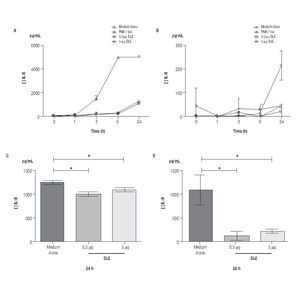
Human Dialyzable Leukocyte Extracts Diminishes secretion of IL-8 and IL-6 in cultured human limbal epithelial cells. To evaluate inflammatory cytokines, hLEC were cultured with two different concentrations of hDLE (0.5 μg/mL or 5 μg/mL); time kinetic-assays were performed at 1h, 3h, 6h and 24h, following cell culture methodology described above. We observed a basal secretion of IL-8 all along the time culture, reaching the highest values at 24h (Figure 2A); but when we compared IL-8 concentration after 24h of cell culture between both, hLEC cultured with medium alone (MA) and hLEC cultured with hDLE, we detected 1.2 - times lower IL-8 concentration in SN from cells cultured with 0.5 μg/mL of hDLE (p = 0.01), and 1.1 - times less IL-8 concentration in hLEC cultured with 5 μg/mL of hDLE (p = 0.03) than in MA (Figure 2C). Similarly results were obtained when we analysed IL-6 during the time-kinetic as-says (Figure 2B). Likewise, when we compared with MA at 24h, we observed 8.4 - times less IL-6 in hLEC supernatants (hDLE (0.5 μg/mL) (p <0.001), and 4.8 times lower IL-6 levels in hLEC supernatants (hDLE, 5 μg/mL) (p <0.001) (Figure 2D). Controls performed with PMA/ion were according as expected (Figure 2, A and B). hDLE did not induce significant changes in secretion of IL1β, TNF-α or IL12p70 in cultured limbal cells, neither with 0.5 μg/mL nor with 5 μg/mL, at any of eva-μg/mL nor any of evaluated times. Results are summarized in Table 1.
◊ Figure 2. Down regulation of IL-8 and IL-6 in hLEC cultured with hDLE. Changes in secretion of IL-8 (A, C) and IL-6 (B, D) were observed in cells cultured with different concentrations of hDLE. After 24h of culture with hDLE (0.5 μg/mL or 5 μg/mL), limbal epithelial cells were able to down-regulate significantly IL-8 (C) and IL-6 (D) secretion. *Statistically significant. (See text for details).
Discussion
Human limbal epithelial cells are stem cells that give rise to corneal epithelium.1-3 hLEC are able to act as a barrier preventing conjunctival epithelial cells from encroaching upon the cornea. During ocular surface infections, epithelial cells could produce inflammatory cytokines trough TLR pathway,4,5 causing conjunctival invasion to the cornea, chronic inflammation, painful cor-neal opacity and neovascularization.5,20 Infectious diseases at the ocular surface have been treated with human dialyzable leukocyte extracts;7-11 clinical-ophthalmological studies have demonstrated that hDLE induce a Th1 systemic response, characterized by an increase of circulating IFN-γ+ T cells.9,11,16 It has been suggested that Th1 systemic response could influence the ocular microenvironment with, until now, an unknown mechanism.11 Because clinical evidence has proposed that hDLE-therapy could be beneficial in ameliorating ocular inflammatory injuries without changes in epithelial regeneration, we sought to determine the cytokines involved in ocular surface inflammation produced by limbal cells.
We performed phenotypic characterization of limbal cells derived from human cadaveric donors; the methodology developed in our laboratory19 was able to obtain highly pure limbal primary cell-cultures, we observed that majority of human limbal-cultured cells were expressing CK19, vimentin, and p63. CK19 is present in all conjunctival and limbal epithelial cells and also in peripheral cor-neal basal cells.21 Vimentin is an intermediate filament that is found in mesenchymal cells, and is expressed in a subpopulation of "transitional cells" in normal limbal tissue, that co-expressed CK19.22 It has been reported by several authors,23-25 that vimentin is always up regulated in cultured cells, these reports are coincident with our results in which we observed high vimentin expression in cultured limbal cells, as shown by the mean fluorescence intensity.p63 is a transcription factor, member of the p53 family, expressed in the nuclei of keratinocytes with proliferative potential.26-28It hasbeen suggested that p63 is a keratinocyte stem cell marker, expressed only in the basal layer of the limbal epithelium with no expression of p63 in the basal cells of the central corneal epithelium (transient amplifying cells).21 The combination of these cell-markers (CK19, vimentin and p63) indicated that the isolated cells from human cadaveric donors were limbal stem cells. Purification and characterization of primary limbal cell-cultures were very important in our study to further assessment of cytokine production in response to human dialyzed leukocyte extracts.
We observed that hDLE induced a down regulation of IL-8 and IL-6 in cultured human limbal epithelial cells, these cells were also unable to secrete of IL-1β or TNF-α after hDLE. During ocular surface injuries, the inflammatory cytokines IL-1β, IL-6, IL-8 and TNF-α could be secreted trough TLRs.29 IL-1β, IL-6, and TNF-α, are cytokines involved in the expression of cell adhesion molecules and secretion of acute phase proteins.30-34 IL-8, also named CXCL8 chemokine, is produced the first 24h after TLR-ligation.35 CXCL8 is responsible to recruit and to activate neutrophils, however at high concentrations IL-8 could inhibit both, neutrophil adhesion to endothelium and extravasation.36 IL-6 may activate endothelial cells to secrete IL-8 and macrophage chemotactic protein (MCP-1).37 MCP-1 production is sustained for several days and its accumulation leads to late monocyte recruitment. The transition from neutrophil to monocyte accumulation in inflammatory cell infiltrate is linked to these changes in chemokine/cytokine pro- duction.38 Secretion of IL-1, IL-6, IL-8 and TNF-α after TLR-ligation, leads to a complex signaling cascade of events including the activation of the transcription factor NF-κB.39 NF-κB has been involved in the control of a wide variety of genes that play critical roles in innate immune responses such as genes encoding cytokines (IL-1, IL-2, IL-6, IL-12, TNF-α, LT-α, LT-β, and GM-CSF), adhesion molecules (ICAM, VCAM, ELAM), acute phase proteins (SAA), and inducible enzymes (iNOS, COX-2).40,41 Interestingly, it has been reported that dialyzable leukocyte extracts previously induced with Sendai virus to produce IFN-γ, are able to reduce TNF-α trough inhibition of NF-κB in the cellular line MT-4;42 similarly, diminished TNF-α secretion was observed in peripheral blood mononuclear cells from HIV patients, stimulated with LPS and with DLE not induced previously with Sendai virus.43 It is possible that the down regulation of IL-8 and IL-6 observed in this study was secondary to direct inhibition of NF-κB activity or throughout inducing naturally inhibitors of NF-κB such as Ikappa-BNS, a TLR-inducible nuclear IkappaB protein, also involved in modulating expression of IL-6 and IL-12.44 If hDLE inhibits NF-κB directly or through IkappaBNS in limbal epithelial cells is not known and needs further investigation. The fact that we observed a diminished secretion of IL-8 and IL-6, without induction of IL-1β and TNF-α in limbal cells cultured with hDLE, could explain from an in vitro system, the clinical observation of inflammatory control at the ocular surface during the treatment with hDLE, however studies in animal models are needed to confirm these data.
Other cytokine investigated in this work was IL-12, a cytokine involved in Th1 differentiation.45 In this context, is well known that hDLE are efficient inductors of IFN-γ (Th1 cytokine);10,11,15 despite limbal cells cultured with hDLE increased IL-12 secretion, this observation was not statistically significant, suggesting that in the experimental model used here, the mechanisms involved in IFN-γ secretion through hDLE would be independent of IL-12. Future studies, including the research of other cytokines involved in the Th1 activation, such as IL-23,46,47 are needed to understand the Th1-axis at ocular surface related with hDLE.
One limitation in our study was the use of phorbol myristrate acetate (PMA) as a positive control, this phorbol ester induces proinflamma-tory cytokines, such as IL-1β, IL-6, IL-8 and TNF-α in multiple epithelial cells;48-50 although, if we want to know the effects of hDLE on proinflamma-tory cytokine secretion through TLR, we need to perform TLR-stimulation assays with their specific ligands; nevertheless this first approach, give us some evidence to identify several anti-inflammatory properties of hDLE in limbal epithelial cells.
Conclusion
The results obtained in this study suggest a down regulation of inflammatory cytokines (IL-8 and IL-6), without induction of IL-1β, TNF-α, and IL-12p70 in human limbal epithelial cells cultured with human dialyzable leukocyte extracts. Despite more studies are needed to better understand the real role of hDLE at the ocular microenvironment in both, health and disease, our results provide a basis to understand some of the clinical effects observed in ocular-infected patients treated with human dialyzable leukocyte extracts.
Acknowledgements
Thanks to Veronica Romero Martinez and Jessica Lopez for their technical assistance. This study was supported in part by the Consejo Nacional de Ciencia y Tecnología (CONACyT) 71291 and by Transfer Factor Project. Robles-Contreras earned her Master Degree in Immunology at the Escuela Nacional de Ciencias Biológicas del Instituto Politécnico Nacional (ENCB-IPN); graduated studies were supported in part by CONACyT and by the Institutional Program of Training Researchers (PIFI, IPN).
Disclosure
The authors declare that they have no financial and personal relationships with other people or organizations that could inappropriately influence this study. This work was presented in part at the Association for Research in Vision and Ophthalmology (ARVO, 2009) (Ft. Lauderdale, CA, USA), Annual Meeting Federation of Clinical Immunology Societies (FOCIS, 2009) (San Francisco, CA, USA); and in full version at the National Congress of Clinical Immunology and Allergy, 2010 (Cole-gio Mexicano de Inmunología Clínica y Alergia, CMICA) (Mazatlan, Sin, MX). This study obtained the second place in the category of Basic Science Research in Immunology (CMICA, 2010)
Corresponding author: Maria C. Jimenez-Martinez MD PhD.
Research Unit, Institute of Ophthalmology Conde de Valenciana. Chimalpopoca 14, Col. Obrera,
C.P. 06800, Mexico City. Telephone: +52 555442 1700, ext. 3212, fax: +52 55 5442 1700, ext. 3207.
E-mail: mcjimenezm@institutodeoftalmologia.org.