Male infertility is characterized by the inability to produce sperm with normal concentration, motility and/or morphology, featuring an abnormal spermatogenesis. The diagnosis of male infertility is accomplished through spermogram.
ObjectivesThe present study aimed to verify the profile of male infertility of patients attended in an assisted human reproduction clinic.
MethodsWe assessed the spermogram report of 196 patients who underwent semen analysis in a private clinic of assisted human reproduction, located in the south of the state of Santa Catarina (Brazil), from 2012 to 2014.
Results32.7% of patients presented normal semen analysis, while 67.3% had some alteration in the report. Among the altered semen, the following diagnoses were found: teratozoospermia (44.7%), oligoasthenoteratozoospermia (20.5%), oligoteratozoospermia (15.9%), azoospermia (7.6%), asthenoteratozoospermia (6.8%), oligozoospermia (2.3%) and asthenozoospermia (2.3%). It was also showed that the sperm volume was modified with advancing age, showing a significant decrease in individuals over 40 years old.
Conclusionsour data revealed teratozoospermia as the most frequent sperm alteration found. Moreover, patients aged greater than or equal to 40 years old presented reduced sperm volume, although the patients’ age did not show correlation with the final diagnosis of the sperm analysis.
A infertilidade masculina é definida pela incapacidade de formar espermatozoides com concentração, morfologia ou motilidade normal, caracterizando uma espermatogênese anormal. O diagnóstico de infertilidade masculina pode ser realizado por meio do espemograma.
ObjetivosO presente estudo buscou verificar o perfil da infertilidade masculina em uma clínica de reprodução humana assistida.
MétodosForam avaliados os laudos de 196 pacientes submetidos ao espermograma em uma clínica particular de reprodução humana assistida, localizada no extremo sul catarinense, no período de 2012 a 2014.
Resultados32,7% dos pacientes apresentaram resultados dentro da normalidade, enquanto que 67,3% apresentaram algum tipo de alteração no espermograma. Entre os exames alterados, os seguintes diagnósticos foram encontrados: teratozoospermia (44,7%), oligoastenoteratozoospermia (20,5%), oligoteratozoospermia (15,9%), azoospermia (7,6%), astenoteratozoospermia (6,8%), oligozoospermia (2,3%) e astenozoospermia (2,3%). Além disso, foi demonstrado que o volume espermático é alterado com a idade, sendo observada uma redução significativa em indivíduos com mais de 40 anos.
Conclusõesnossos resultados revelaram a teratozoospermia como a alteração seminal mais frequente na população estudada. Além disso, pacientes com idade maior ou igual a 40 anos apresentaram o volume espermático reduzido, embora a idade dos pacientes não se correlacione com o diagnostico final do espermograma.
Infertility is defined as the inability of a sexually active couple, without the use of contraceptive methods, to obtain a pregnancy within one year. It is an important pathological condition that affects around 8–15% of couples regardless of socioeconomic and cultural factors. Moreover, it is not characterized as a permanent disability to have children.1
The male infertility, in turn, is characterized by the failure to produce sperm with regular concentration, morphology and/or motility, consisting in an abnormal spermatogenesis.2–4 Lately, the correlation of males’ age and infertility has been extensively studied. Recent researches have shown that the higher the paternal age, more likely to have disorders of production and quality of the sperm.5 According to Telõken, Badalotti and Palka (1999), there isn’t an age threshold to the male fertility, although a decrease can be seen since 40 years old.6 In addition, factors like urogenital infection, exposure to toxics substances and vascular diseases can contribute to changes in the seminal parameters, having many consequences as: difficulties in the embryo formation, increased risk of early pregnancy loss and a higher probability to develop genetic syndromes or other diseases.5 Thus, it is important to note that male infertility is multifactorial, presenting manyetiologies.7
As the first choice test, the semen analysis, although not sophisticated, is used to determine if a seminal sample are in accordance with WHO (World Health Organization) reference values.4 The diagnostic of male infertility depends on a descriptive evaluation of the ejaculate, with emphasis in the concentration, motility and morphology of the sperm, being these parameters of great clinical relevance. The changes detected in the semen analysis include: azoospermia, total absence of spermatozoa in the ejaculate; oligozoospermia, reduced number of sperm; teratozoospermia, presence of abnormal morphologies in the sperm; asthenozoospermia, low sperm motility and; necrozoospermia, high percentage of immotile spermatozoa.4,8 In some cases, a combination of two or more alternations is found.9
Considering that only few data about male infertility in the south of Santa Catarina are available, the present study aimed to assess the male infertility profile in a private human assisted reproduction clinic from 2012 to 2014.
Materials and methodsExperimental design and data collectionTo evaluate the profile of male infertility, data from the spermogram reports were collected in a private human assisted reproduction clinic in Criciúma, Santa Catarina (Brazil), which attends patients from that city and from neighboring towns. Only data from patients who carried out the semen analysis in the studied clinic and issued from 2012 to 2014 were included in the. In addition to the quantitative and qualitative features found in the sperm analysis is, the patients’ age was evaluated.
Statistical analysisThe sample size calculation was performed according to Medronho et al.10 and Barbetta,11 resulting in a minimum sample size of 196 reports, that were chosen randomly from a total of 400 spermograms report,. Data were analyzed with the software Statistical Package for the Social Sciences (SPSS), version 22.0. A statistical significance level of α=0.05 and a confidence interval of 95% were applied to all tests. The spermogram qualitative variables were expressed as percentage and frequency while the quantitative ones were expressed as mean±standard error of the mean (SEM).
The age of the patients was expressed as mean±SEM, whereas its distribution was evaluated for normality by Kolmogorov–Smirnov test. The comparison of the age mean among the outcome variable categories (dichotomized) was performed with Student's t test preceded by the Levene test. In addition, the age mean was also compared among the final diagnoses of sperm analysis through one-way ANOVA test.
The existence of an association between age (dichotomized) and the diagnosis (normal or abnormal) was assessed by Pearson's Chi-squared test and the Mann–Whitney U test was used to compare the quantitative characteristics of semen with dichotomized age (40 years).
Ethic concernsThis research was conducted in accordance with the Helsinki Declaration, as revised in 2008. The subject's privacy and confidentiality were guaranteed and the study was approved by the Universidade do Extremo Sul Catarinense (UNESC) Research Ethics Committees.
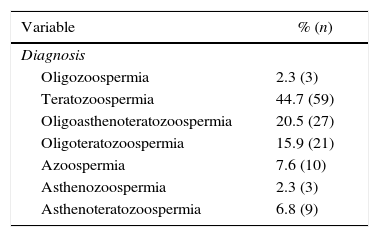
ResultsTeratozoospermia was the most frequent diagnosis among altered reportsFrom 196 reports analyzed, 32.7% (n=64) presented normal results (normozoospermia) while 67.3% (n=132) were abnormal. Among the 132 cases, the following diagnoses were found: 44.7% (n=59) with teratozoospermia, 20.5% (n=27) with oligoasthenoteratozoospermia, 15.9% (n=21) with oligoteratozoospermia, 7.6% (n=10) with azoospermia, 6.8% (n=9) with asthenoteratozoospermia, 2.3% (n=3) with oligozoospermia and 2.3% (n=3) with asthenozoospermia (Table 1). Furthermore, Table 2 shows the mean of the semen quantitative variables: concentration, volume, normal sperm morphology and progressive motility. Compared to the WHO reference values, lower results were found in 30.6% of the cases (n=60) regarding to the concentration, 11.2% (n=22) to the volume, 71.4% (n=140) to the morphology and 25.0% (n=49) to the motility (Table 2).
Frequency and percentage of the changes found in the sperm analysis.
| Variable | % (n) |
|---|---|
| Diagnosis | |
| Oligozoospermia | 2.3 (3) |
| Teratozoospermia | 44.7 (59) |
| Oligoasthenoteratozoospermia | 20.5 (27) |
| Oligoteratozoospermia | 15.9 (21) |
| Azoospermia | 7.6 (10) |
| Asthenozoospermia | 2.3 (3) |
| Asthenoteratozoospermia | 6.8 (9) |
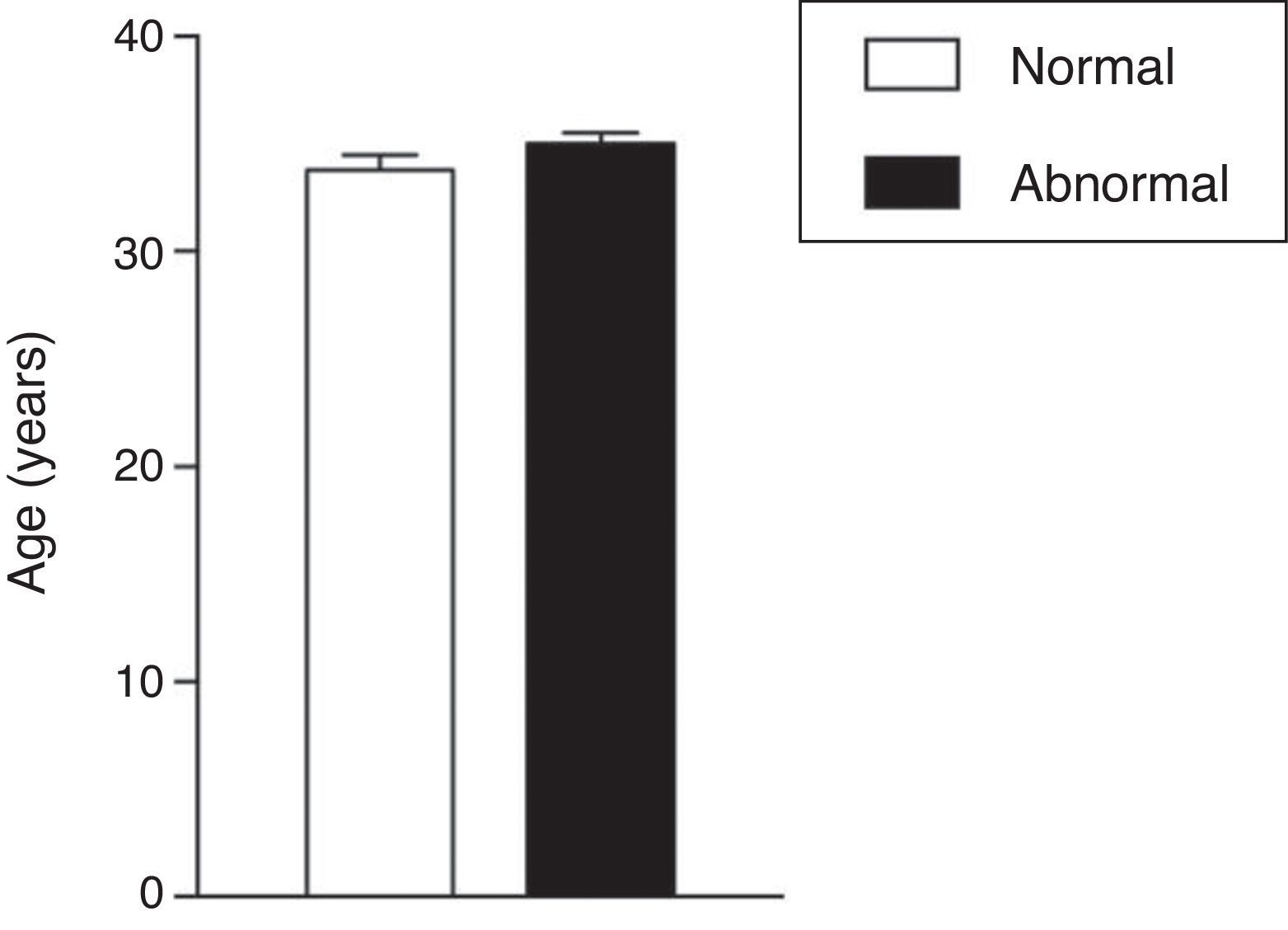
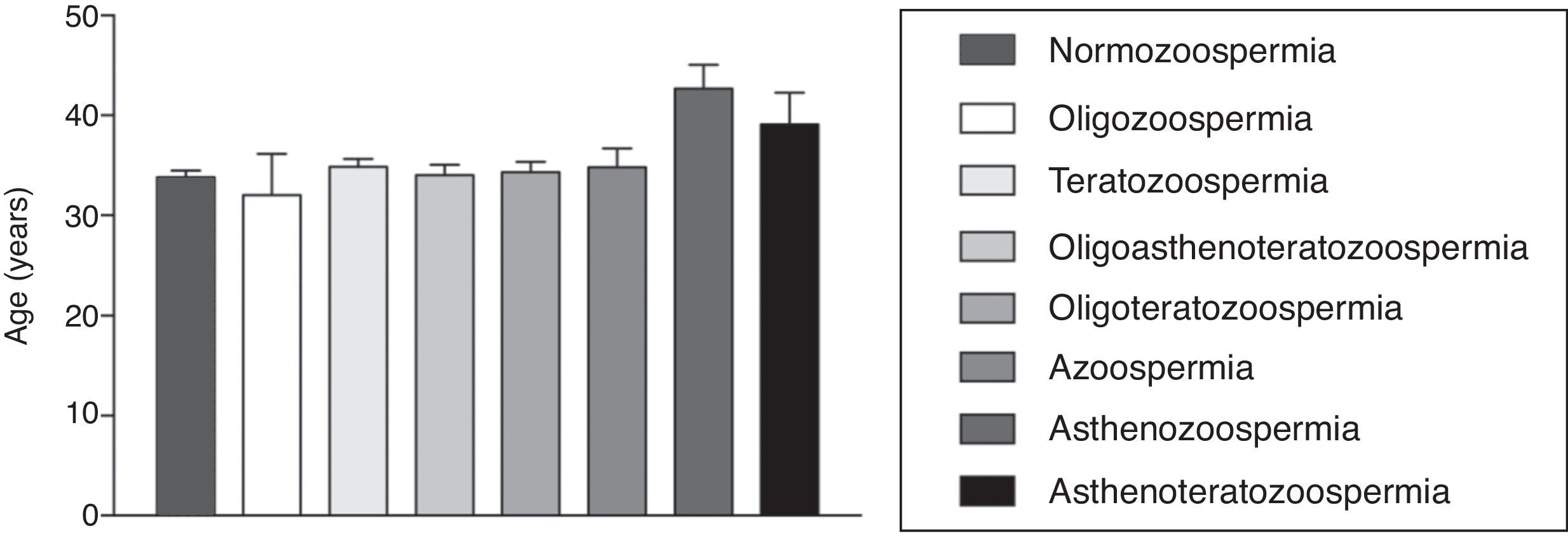
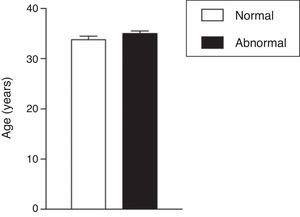
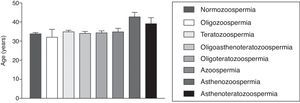
The age mean of patients who underwent the spermogram was 34.61±0.43 years old and 33.2% of these individuals were in the age group of 30 to 34 years old, being this group the most prevalent in the patients with abnormal sperm analysis reports (data not shown). There was no statistically significant difference between the age mean of patients with normal and abnormal semen analysis (33.80±0.38 years for patients with normal results vs. 35.00±0.43 years for patients with abnormal test; p=0.178, Student's t test; Fig. 1). Also, the age mean was similar for the different types of abnormalities found (p=0.0617 in one-way ANOVA; Fig. 2).
According to the literature data, patients aged greater than or equal to 40 years old show a decrease in the fertility rate. Thus, our study compared the qualitative and quantitative variables assessed in the spermogram with the age dichotomized (smaller and greater than or equal to 40 years old).
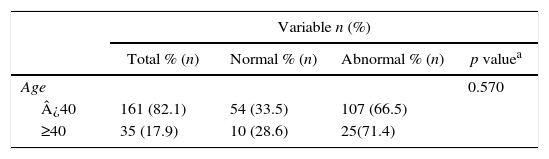
Among the reports analyzed, 82.1% of men are younger than 40 years old, being that 33.5% of these patients presented normal semen analysis and 66.5% abnormal. Regarding men older than 40 years old, 28.6% showed results within the reference values and 71.4% presented some disturbance in the semen analysis (Table 3). These data indicate that male infertility rate does not differ with respect to age above or below 40 years (p=0.570, Chi-square test Pearson).
Correlation between dichotomized age and normal or abnormal outcomes.
| Variable n (%) | ||||
|---|---|---|---|---|
| Total % (n) | Normal % (n) | Abnormal % (n) | p valuea | |
| Age | 0.570 | |||
| ¿40 | 161 (82.1) | 54 (33.5) | 107 (66.5) | |
| ≥40 | 35 (17.9) | 10 (28.6) | 25(71.4) | |
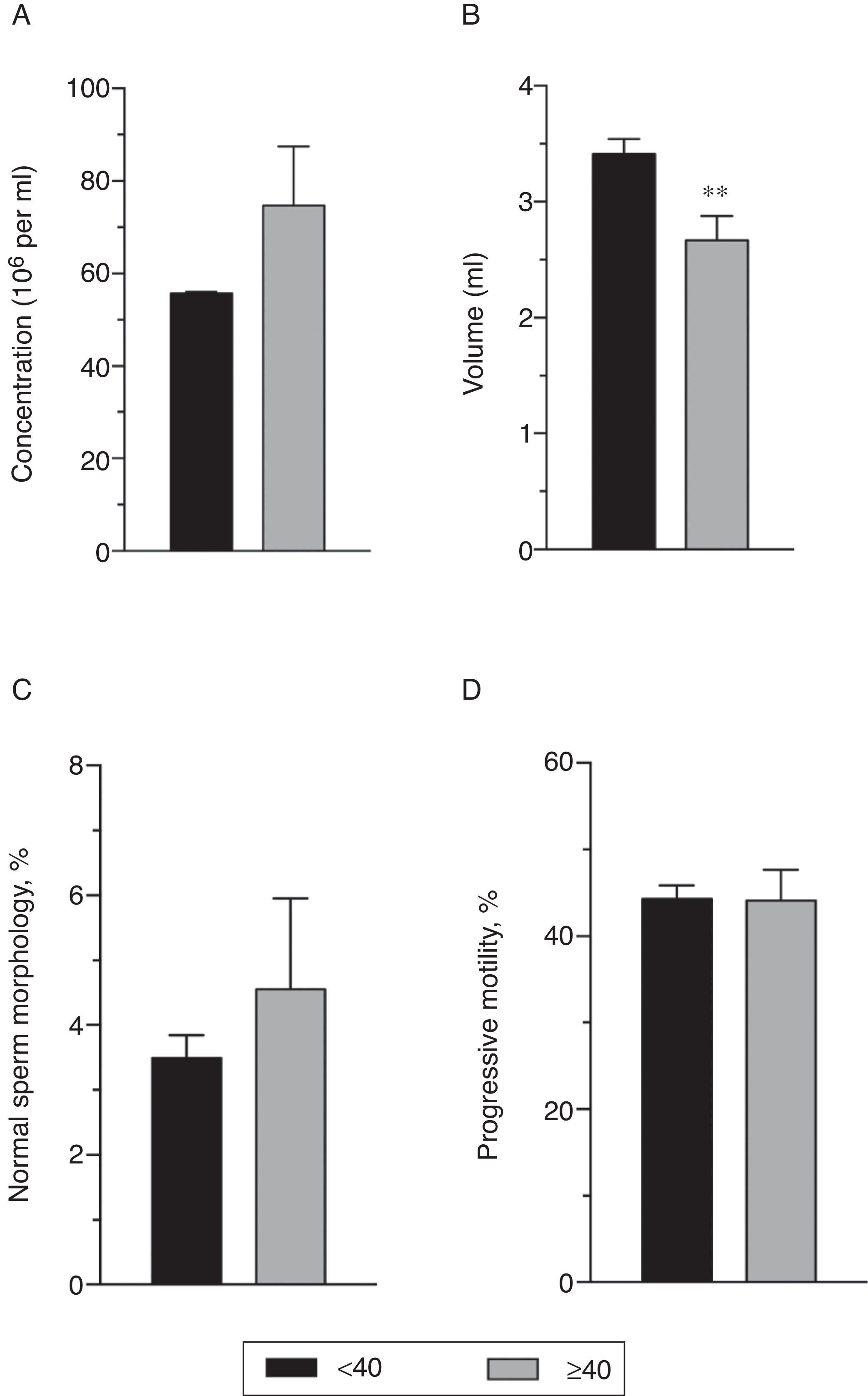
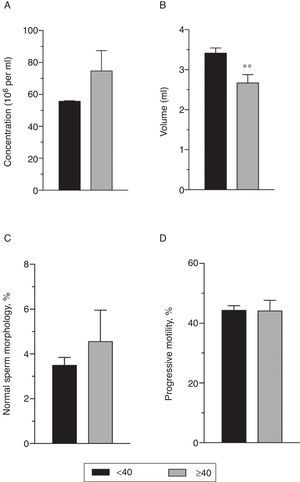
On the other hand, the Mann–Whitney U test revealed a decrease in the sperm volume in patients aged ≥40 years old (p=0.007). The other quantitative variables did not show statistically significant difference regarding the dichotomized age (Fig. 3).
Comparison between quantitative variables of the sperm analysis and the dichotomized age. Data expressed as mean±SEM. (A) Sperm concentration (million/ml); (B) Spermatic volume (ml). **p=0.007 vs. <40 years in Mann–Whitney U test; (C) Normal sperm morphology (%) and (D) Progressive Motility (%).
Studies show that one in ten couples, worldwide, are affectedbyinfertility.12 World statistics estimates that about 15–20% of the population who want to father a child, has some kind of disturbance that may result in a diagnosis of infertility.13 Men, alone, are responsible for about 30–40% of cases in which the couple is struggling to get pregnant,14 plus the cases combined to female causes, which reach 20%. Therefore, the male factor is present in at least 55% of the cases of marital infertility.3 Despite of scientists effort, 10% of infertility cases remain unclear.15 Thus, it is highly relevant, for epidemiological purposes, to identify the male infertility profile and, in this sense, the sperm analysis is the gold standard to diagnose it.16 Hence, we evaluated the spermogram reports, carried out from 2012 to 2014, to describe the male infertility profile in a human assisted reproduction clinic of Santa Catarina.
Our study showed a patients age mean of 34.61±5.85 years old, being that the major age group was among 30–34 years old, corresponding to 33.2% of the patients. Similar results were found by Castro et al.,17 that found an age mean of 32.47±8:39 years and the major age group among 25 and 34 years old (52.4%). In addition, Faria et al.18 reported that patients age ranged from 24 to 54 years old, with a predominance of individuals aged among 36 to 44 years old (45%). Another study has also shown an age mean of 37±7.9 years old, with 56% of patients aged among 30 and 39 years old.5
Of the 196 spermogram reports assessed in our study, 67.3% presented some kind of change. These data are consistent with Bashed et al.19 that described abnormalities in sperm analysis of 60% of men surveyed. Moreover, Ríos and colleagues20 have also shown that 54.9% of assessed subjects presented at least one variable of the semen analysis beneath the reference values. Turek and Pera,21 on the other hand, reported that 55% of men studied showed sperm with normal parameters.
In the present study, the most frequent diagnosis, among the abnormal reports, was teratozoospermia, corresponding to 44.7% of the cases. Some studies showed a higher frequency of azoospermia (40%) followed by oligozoospermia (34%)20 and asthenozoospermia (26%),21 while teratozoospermia was found only in 1% of patients assessed.19,21 Sohrabvandet al.,22 in turn, found a higher prevalence of oligozoospermia (23%) followed by asthenozoospermia (17%), while 2% of the patients presented teratozoospermia. In addition, Turek and Pera21 showed oligozoospermia diagnosis in 8% of cases. Furthermore, Sigman, Jonathan, Jarow23 found that 14% of studied men showed azoospermia, 6% asthenozoospermia, 4% oligozoospermia and 4% teratospermia. Our data showed that 5.1% of patients were diagnosed to azoospermia, 1.5% to oligozoospermia and 1.5% to asthenozoospermia.
These data indicate that there is a great variability in the frequency of different male infertility diagnoses. Studies have shown that there are many risk factors that are directly or indirectly related to the causes of male infertility. These risks may be related to age and to certain environmental factors and lifestyles, such as of occupational exposure, alcohol and caffeinated beverages intake, smoking and psychological stress.19 Moreover, there are cases of infertility related to genetic and idiopathic causes.24 Furthermore, it was also demonstrated that the risk factors for male infertility can vary among different countries and regions.19 which explains the variability seen among the studies.
Besides, studies show that the combination of two or more changes in semen is usual, ranging from 49% to 54% of the cases of male infertility.22,23
Our study found this combination in 29.1% of the cases, which correspond to oligoasthenoteratozoospermia (13.8%), oligoteratozoospermia (10.7%) and asthenoteratozoospermia (4.6%).
Our study also evaluated the mean of the quantitative variables found in the semen analysis of the patients. The volume mean observed was 3.28±0.12ml, which agrees with previousdata.5,17 We also noted a concentration mean of 59.08±4.66 million spermatozoa per milliliter of semen, a normal sperm morphology mean of 3.28±0.38% and a progressive motility mean of 44.30±1.42%. Cavalcante et al.5 found a concentration mean of 61.4±66.4 million/ml, a normal sperm morphology mean of 11.2±6.6% and a progressive l motility mean of 44.7±19.4% of the total spermatozoa sample, indicating a difference in the sperm morphology data. In addition, another study saw a concentration mean of 51.83±47.20 millions/ml and a progressive motility mean of 58.60±35.97%.17 These data confirm the variability found in the infertility diagnosis of different studies, reinforcing the possibility of different risk factors and studied regions influence on infertility profile.
Studies also indicate an increased age as a risk factor for male infertility. We noticed a decrease in the semen volume in men among 40 and 50 years old. Moreover, researches demonstrate a decrease in the progressive motility and normal sperm morphology with advancing age. On the other hand, the information on sperm concentration regarding to aging are controversial, not always indicating a decrease in this parameter.6,25
The present study also compared the age mean of patients with normal sperm analysis to men with abnormal results and no statistically significant difference was verified. Furthermore, the age mean for each sperm alteration identified was not significantly different among them.
We also compared the volume, progressive motility, normal morphology and concentration means of sperm of patients younger than 40 years old with patients older than 40 years old (or equal to). There was a statistically significant decrease in seminal volume in patients from 40 years old, while in the other parameters no significant differences among groups was seen. Besides the seminal volume, Castro et al.17 demonstrated correlation of aging with decreased concentration and motility of spermatozoa.
On the other hand, a Brazilian retrospective study showed that only the volume was reduced with advancing age, which is accordance with our findings. The volume of semen samples was normal in 73.6% of subjects; being below 2ml in 18.4% of patients and above 5ml in 5% of assessed samples,5 whereas in our studies, 174 (88.8%) reports showed normal volume when compared to the WHO reference values. However, 22 patients (11.2%) presented a lower volume.
Conflicting results from different studies may be due to cultural, environmental, economic and social factors of each country, affecting, therefore, the epidemiological characterization of infertility.21 It was described that infertility patterns in developing countries, differ from those in developed countries, beyond the infertility incidence by external agents be higher in countries development.19
In summary, our results demonstrated that 32.7% (n=64) of the total reports showed a normal sperm analysis, whereas 67.3% (n=132) presented some altered condition. Among the changes found, teratozoospermia, was the most prevalent, corresponding to 44.7% (n=59) of the cases. We also showed an important effect of age on the sperm volume, which presents reduced in patients aged 40 years old or more.
Conflicts of interestThe authors declare no conflicts of interests.
We would like to thank all the collaborators of the assisted human reproduction clinic who authorized and facilitated the data's collection.