The main objective of individualization of treatment in IVF is to offer every single woman the best treatment tailored to her own unique characteristics, thus maximizing the chances of pregnancy and eliminating the iatrogenic and avoidable risks resulting from ovarian stimulation. Personalization of treatment in IVF should be based on the prediction of ovarian response.
ObjectiveTo summarize the predictive ability of ovarian reserve markers, and the therapeutic strategies that have been proposed in IVF after this prediction.
MethodsA systematic review of the existing literature was performed by searching Medline, LILACS, SciELO and Pubmed, for publications related to ovarian reserve markers and their incorporation into controlled ovarian stimulation (COS) protocols in IVF.
Results251 articles were found. Ten articles published between 2010 and 2015 were selected.
ConclusionAntral follicle count (AFC) and anti-Mullerian hormone (AMH), the most sensitive markers of ovarian reserve, are ideal in planning personalized COS protocols. These markers permit prediction of the ovarian response with reliable accuracy. Following the categorization of expected ovarian response clinicians can adopt tailored therapeutic strategies for each patient.
O principal objetivo da individualização do tratamento na fertilização in vitro é oferecer a cada mulher o melhor tratamento sob medida para suas próprias características únicas, maximizar, assim, as chances de gravidez e eliminar os riscos de iatrogenia durante a estimulação ovariana. A personalização do tratamento na fertilização in vitro deve basear-se na predição da resposta ovariana.
ObjetivoAvaliar o uso de marcadores da reserva ovariana para individualização da dose inicial do FSH nos ciclos de FIV.
MétodosRevisão sistemática da literatura feita por meio de pesquisa Medline, Lilacs, SciELO e PubMed, para publicações relacionadas com marcadores reserva ovariana e sua incorporação, estimulação ovariana (COS) e protocolos controlados em fertilização in vitro.
ResultadosForam achados 251 artigos. Foram selecionados dez artigos publicados entre 2010 e 2015.
ConclusãoContagem de folículos antrais (AFC) e hormônio anti-Müulleriano (AMH), os marcadores mais sensíveis da reserva ovariana, são ideais no planejamento de protocolos individualizados. Esses marcadores permitem previsão da resposta ovariana com confiança. De acordo com a resposta ovariana esperada, os clínicos podem adotar estratégias terapêuticas sob medida para cada paciente.
It is well established that successful IVF and embryo transfer requires both stimulation of the ovary and suppression of the pituitary. Thus, exogenous gonadotropins and gonadotrophin-releasing hormone (GnRH) analogues are considered the hormones required to maximize IVF success.1
The daily dose of gonadotrophin administered in assisted reproduction technology may be fixed but usually it is progressively increased or tapered according to the given patient's response.2
A key issue in the management of cycles is defining the optimal starting dose of FSH for each patient in order to obtain the optimization of response and outcomes whilst minimizing the risks.3–5
In this article we discuss the use of the most recently identified markers of ovarian reserve, to categorize women based on their anticipated ovarian response. The marker-based strategy of assessing ovarian reserve in women in order to select the ideal therapeutic approach in IVF is reviewed.
MethodsA systematic review was conducted of studies published from January 2010 to December 2015 in English, Portuguese and Spanish. The following databases were consulted: Medical Literature Analysis and Retrieval System Online (MEDLINE), Literature Latin American and Caribbean (LILACS), Scientific Electronic Library Online (SciELO), and US National Library of Medicine (PubMed).
The descriptors used were: anti-Mullerian hormone (AMH), antral follicles, antral follicle count (AFC), ovarian reserve and several synonyms of IVF and ICSI. Among the studies identified, prospective studies, systematic reviews and retrospective studies that addressed ovarian reserve markers as predictors of ovarian response and individualized optimal dose of follicle stimulating hormone (FSH) to reduce inappropriate responses in an IVF cycle were selected. Inclusion criteria were studies with a sample composed of women <40 years of age with regular menstrual cycles, without ovarian anatomical changes and with causes of infertility treated by assisted reproduction techniques (ART).
The studies were selected independently and blindly by two authors according to the inclusion and exclusion criteria. Where there was disagreement between the two authors, the opinion of a third author was employed.
Two hundred and fifty-one published articles were identified from the descriptors and filters used. One hundred and one articles were excluded by the title, by reading the abstracts, or because of repetition in the databases, and 150 articles were selected that related ovarian reserve markers as predictors of ovarian response to individualized optimal dose of FSH to reduce inappropriate responses.
From these 150 articles, six that respected the inclusion criteria defined for this study were selected. The reference lists of the selected articles were analyzed to investigate the existence of new articles addressing the topic that could be incorporated.
Four more articles fitting the proposed inclusion criteria were included, making a total of 10 articles analyzed in this study.
Fig. 1 shows the flow chart summarizing the strategy adopted to identify and include the studies. Because this study used only data published in the literature, approval by an institutional review board was not required.
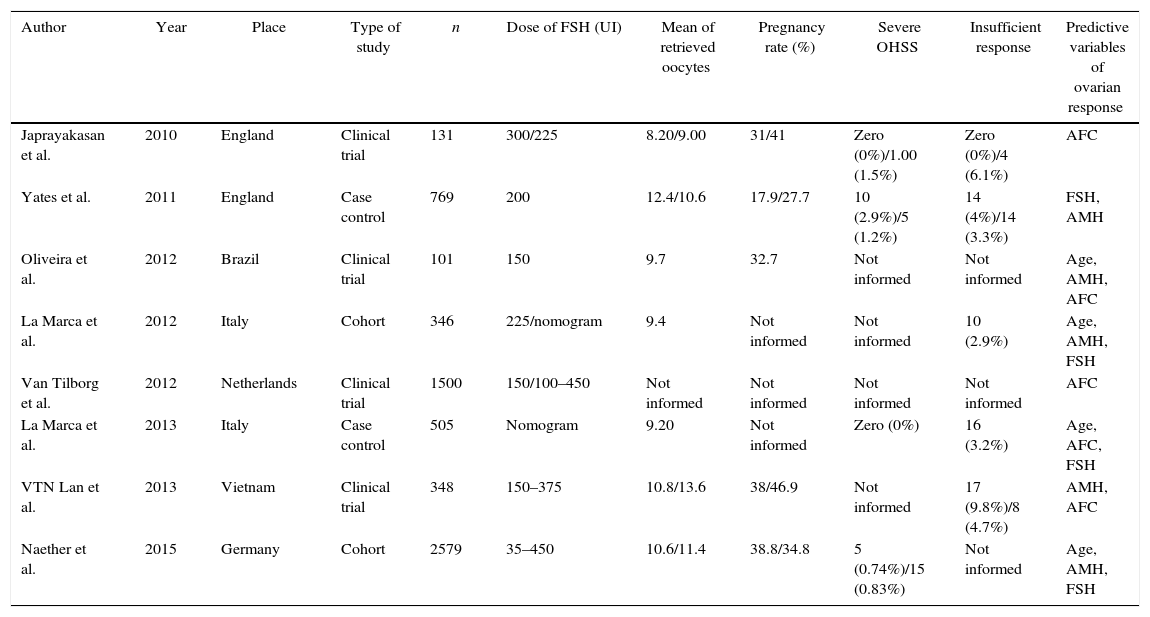
ResultsUsing the data from the articles, Table 1 was constructed for comparative analysis. Six of the ten articles analyzed were prospective studies dealing with clinical trials and cohort studies, and two was case–control studies with retrospective design. Furthermore, two systematic reviews were included in this study.
Summary of data found in the systematic review.
| Author | Year | Place | Type of study | n | Dose of FSH (UI) | Mean of retrieved oocytes | Pregnancy rate (%) | Severe OHSS | Insufficient response | Predictive variables of ovarian response |
|---|---|---|---|---|---|---|---|---|---|---|
| Japrayakasan et al. | 2010 | England | Clinical trial | 131 | 300/225 | 8.20/9.00 | 31/41 | Zero (0%)/1.00 (1.5%) | Zero (0%)/4 (6.1%) | AFC |
| Yates et al. | 2011 | England | Case control | 769 | 200 | 12.4/10.6 | 17.9/27.7 | 10 (2.9%)/5 (1.2%) | 14 (4%)/14 (3.3%) | FSH, AMH |
| Oliveira et al. | 2012 | Brazil | Clinical trial | 101 | 150 | 9.7 | 32.7 | Not informed | Not informed | Age, AMH, AFC |
| La Marca et al. | 2012 | Italy | Cohort | 346 | 225/nomogram | 9.4 | Not informed | Not informed | 10 (2.9%) | Age, AMH, FSH |
| Van Tilborg et al. | 2012 | Netherlands | Clinical trial | 1500 | 150/100–450 | Not informed | Not informed | Not informed | Not informed | AFC |
| La Marca et al. | 2013 | Italy | Case control | 505 | Nomogram | 9.20 | Not informed | Zero (0%) | 16 (3.2%) | Age, AFC, FSH |
| VTN Lan et al. | 2013 | Vietnam | Clinical trial | 348 | 150–375 | 10.8/13.6 | 38/46.9 | Not informed | 17 (9.8%)/8 (4.7%) | AMH, AFC |
| Naether et al. | 2015 | Germany | Cohort | 2579 | 35–450 | 10.6/11.4 | 38.8/34.8 | 5 (0.74%)/15 (0.83%) | Not informed | Age, AMH, FSH |
AFC, antral follicle count; AMH, anti-Mullerian hormone; FSH, follicle stimulating hormone.
The earliest study was published in 2010 by Japrayakasan et al., in England, while the most recent were published in 2015 by Castro et al., and Naether et al., in Brasil and Germany, respectively. This study conducted in Germany, has the largest sample, with 2579 patients.
The smaller study, with 131 women, was conducted in the UK by Jayaprakasan et al., in 2010.
DiscussionThe main goal of individualized treatment in IVF cycles is to provide every patient with therapy based on their unique characteristics, thereby permitting a greater chance of success with lower risks from ovarian stimulation.6–8
A key factor determining the outcome of COS and subsequent IVF outcome is selection of the gonadotrophin starting dose. The need for individualizing gonadotrophin dosage derives from the assumption that variability in the functional ovarian reserve (the pool of recruitable follicles) is very wide and consequently a standard fixed dose of gonadotrophin may not be suitable for all women.
Correct individualization of the gonadotrophin start dose is an extremely important clinical decision.9,10
The correct individualization of treatment protocols in IVF should be based on the correct prediction of ovarian response especially the extremes, namely poor and hyper response. The aim is then to choose the ideal treatment protocol according to this prediction. The prediction of a poor or hyper response also allows clinicians to give women more accurate information regarding the likelihood of these scenarios occurring during their IVF cycle.11
Patients may receive more accurate information on possible protracted treatment, cycle cancellation OHSS, treatment burden and reduced success. Finally, if personalization is based on the accurate prediction of ovarian response, then the prediction of ovarian response should be based on the most sensitive markers of ovarian reserve.12,13
Lan et al. compared the efficacy and safety of two simple dosing algorithms, one based on AMH and the other on the AFC, to determine the starting dose rFSH for ovarian stimulation in 348 women. This pilot study, concluded that, with subtle differences, both AMH and AFC appear to have the ability to predict poor ovarian response and guide the starting dose of rFSH. Therefore, other factors might influence the choice of test.14
Advantages of AMH include intracycle stability and the fact that concentrations can be determined from blood obtained during routine IVF testing. In contrast, AFC needs to be determined early in the follicular phase of the cycle by a skilled ultrasound operator and the measurement requires standardization.14
Although AFC is probably one of the most widely used markers of ovarian reserve in the context of IVF, it is surprising that there is currently a lack in the literature of simple models based on AFC as a single variable dictating the treatment strategy.15
A large RCT, developed by Van Tilborg et al., is ongoing in the Netherlands aimed at comparing the gonadotrophin starting dose for ovarian stimulation in IVF dictated by AFC versus a standard gonadotrophin dose. In this study women are categorized into groups based on AFC and randomized to receiving either an individualized or standard gonadotrophin dose.
The objectives of this study are the success rates in terms of the live birth rate and the evaluation of cost-effectiveness of the individualization of the gonadotrophin dose on the basis of AFC.16
In 2010, Jayaprakasan et al. used AFC as a predictor of ovarian response to compare fixed doses of gonadotropins (225 and 300IU) and did not observe a significant difference in the number of oocytes retrieved in women undergoing these doses during an IVF cycle.17
Regarding the use and efficacy of serum AMH levels in tailored treatment, a recent retrospective study studies have been published comparing the study group undergoing a therapeutic protocol based on AMH levels versus a control group undergoing treatment based on pretreatment FSH levels confirmed that tailored treatment based on AMH reduced the incidence of OHSS.
Moreover, the study showed a significant increase in both pregnancy (17.9 versus 27.7%) and live birth rates (15.9 versus 23.9%) in the study group compared with the control group. This seems to confirm that individualized therapy can improve IVF outcomes.
Finally, and not least importantly, the study group also showed an important reduction of costs probably due to a reduced incidence of OHSS and drug consumption.18
An easy to use algorithm to calculate the gonadotrophin dose based on AFC has recently been published.
This model, although interesting, requires validation in an independent cohort as it is based on a retrospective analysis. The multivariate regression analysis showed that independent predictors of ovarian response expressed in terms of retrieved oocytes were age, AFC and Day 3 serum FSH, with AFC being the most significant predictor. The nomogram calculated the gonadotrophin dose based on the age of the woman, Day 3 serum FSH level and AFC.19
A similar nomogram based on AMH had previously been developed by the same group. The choice of developing two different nomograms based on AMH or AFC followed the recognition that clinicians usually rely on measuring one marker of ovarian response, either AFC or AMH.
The multivariate AMH based model was developed on 346 women undergoing ovarian stimulation with the same protocol (the long GnRH agonist standard protocol) and the same dose of gonadotrophin. The variables analyzed as possible predictors of ovarian response to stimulation were Day 3 serum FSH, estradiol, AMH, BMI and smoking status.
A multivariate regression analysis showed that independent predictors of ovarian response, expressed in terms of retrieved oocytes, were age, AMH and Day 3 serum FSH with AMH being the most significant predictor. According to the model, for women of similar age, the number of retrieved oocytes per unit of gonadotrophin was reduced with decreasing levels of basal AMH and increasing levels of Day 3 serum FSH.
The multivariate model was the basis of a nomogram for the selection of the most appropriate gonadotrophin starting dose.20 As with the AFC-based nomogram, the model incorporating AMH needs to be validated in an external and independent population before adoption into routine clinical practice.19,20
Oliveira et al. recently presented a new ovarian response prediction index (ORPI), based on AMH levels, AFC and age, and set-up a study to verify whether it could be a reliable predictor of the ovarian stimulation response. A total of 101 patients were enrolled in the intracytoplasmic sperm injection (ICSI) programme were included.
The ORPI values were calculated by multiplying the AMH level by the number of antral follicles, and the result was divided by the age (years) of the patient (ORPI=AMH×AFC/patient age).
The regression analysis demonstrated significant (p<0.0001) positive correlations between the ORPI and the total number of oocytes collected. The ORPI exhibited an excellent ability to predict a low ovarian response and a good ability to predict an excessive response and the occurrence of pregnancy in infertile women.
The ORPI might be used to improve cost benefit ratio of ovarian stimulation regiments by guiding the selection of medication and by modulating the doses and regimens according to the actual needs of the patients.21
Naether et al. reported a postmarketing surveillance survey conducted to investigate the utility of the CONsistency in r-FSH Starting dOses for individualized tReatmenT (CONSORT) calculator for individualizing recombinant human follicle stimulating hormone (r-hFSH) starting doses for controlled ovarian stimulation (COS) in routine clinical practice.
In this large observational study, the starting doses of r-hFSH for COS recommended by physicians in routine clinical practice were generally higher than the CONSORT-calculated doses.
In addition, most patients received an actual starting dose of r-hFSH that was higher than the CONSORT-calculated dose, suggesting a lack of trust by the physician in the CONSORT-calculated dose.
Further research to evaluate the full clinical impact of dosing algorithms on safety and efficacy during COS is warranted.22
ConclusionThe availability of new markers of ovarian reserve, the improvement in methodology for their measurement and the huge amount of clinical data have supported the view that individualization in IVF is the way forward. Ovarian response in IVF is a complex puzzle for which we now know the most important pieces.
The correct measurement of markers of ovarian reserve allows a scientific estimate of the pool of follicles that potentially respond to ovarian stimulation.
Published studies indicate an important role for both AFC and AMH in the prediction of the extremes of ovarian response and for enabling the subsequent individualization of a therapeutic strategy.
This is the basis for the correct selection of women for use of the different GnRH analogues and, for the fine tuning of the gonadotrophin dose.
The ultimate goal would be the selection of an effective protocol for ovarian stimulation which has to be well balanced between the risk of maximal and suboptimal ovarian response. The benefits of a personalized therapy may include reduced incidence of risks and dropout as well as a reduced treatment burden.
Nevertheless, a clear definition for modality of a correct application of the individualized therapy is still required to optimize efficacy and daily clinical management.
Conflicts of interestThe authors declare no conflicts of interest.