To compare the effect of adding short course follicular phase dexamethazone to clomiphene citrate versus increasing clomiphene citrate dose in cases of resistance.
Materials and methodsThe study was conducted on 85 with polycystic ovary syndrome who were resistant to 150mg clomiphene citrate daily for 5 days for 3 months and were attending El Shatby Maternity University Hospital in the period between September 2011 and May 2013. These patients were randomly allocated by closed envelope method into one of the two groups: Group I: 44 patients received 200mg daily of clomiphene citrate from day 3 and for 5 days together with 2mg dexamethazone daily from day 3 and for 10 days. Group II: 41 patients received 200mg daily of clomiphene from day 3 and for 5 days.
ResultsWe found that a significant higher rate of ovulation was reported in the dexamethazone group compared to clomphine citrate only group, endometrial thickness was more favorable in dexamethazone group than the other group, mid luteal serum progesterone was a higher level in dexamesathone group compared to the other group and serum pregnancy rate was higher in dexamethazone group than the other group.
ConclusionAlthough the mechanism underlying the beneficial effects of dexamesathone is not exactly understood, it is apparent from our data that dexamesathone therapy during the follicular phase can enhance follicular development and ovulation. Dexamethazone (corticosteroids) therapy combined with clomphine citrate can improve folliculogenesis, ovulation, and pregnancy rate in clomphine citrate resistant polycystic ovary syndrome.
Comparar o efeito da adição de dexametasona em curso de curta duração ao citrato de clomifeno na fase folicular versus aumento da dose de citrato de clomifeno em casos de resistência.
Materiais e métodosO estudo foi realizado em 85 mulheres com síndrome do ovário policístico e resistentes à medicação com citrato de clomifeno 150mg/dia durante 5 dias por 3 meses e que se encontravam em tratamento na Maternidade do Hospital Universitário El Shatby no período entre setembro de 2011 e maio de 2013. Essas pacientes foram designadas randomicamente pelo método do envelope fechado em um entre dois grupos: Grupo I: 44 pacientes medicadas com citrato de clomifeno 200mg/dia a partir do terceiro dia e durante 5 dias, juntamente com dexametasona 2mg/dia a partir do terceiro dia e durante 10 dias; Grupo II: 41 pacientes medicadas com citrato de clomifeno 200mg/dia a partir do terceiro dia e durante 5 dias.
ResultadosConstatamos que um percentual significativamente mais elevado de ovulações foi informado no grupo de dexametasona, em comparação com o grupo medicado exclusivamente com citrato de clomifeno; em comparação com o outro grupo, no grupo das mulheres tratadas com dexametasona a espessura do endométrio se revelou mais favorável, foram observados níveis séricos mais altos de progesterona na metade da fase lútea, e o percentual de gestação sérica foi mais elevado.
ConclusãoEmbora o mecanismo subjacente aos efeitos benéficos obtidos com o uso de dexametasona não tenha ainda sido completamente esclarecido, fica evidente, com base em nossos dados, que o tratamento com dexametasona durante a fase folicular pode melhorar o desenvolvimento dos folículos e a ovulação. A terapia com dexametasona (um corticosteroide) em combinação com citrato de clomifeno pode melhorar os percentuais de foliculogênese, de ovulação e de gestações em mulheres com SOPC resistentes ao citrato de clomifeno.
Chronic anovulation is one of the examples of ovulatory dysfunction. The polycystic ovary syndrome (PCOS) is the most obvious and common heterogeneous endocrine condition associated with chronic anovulation affecting 4–6% of reproductive age group.1 It is one of the examples of eugonadotropic eu-estrogenic anovulation, as it represents 75–80% of all ovulatory disorders. It was discovered by both Stein and Leventhal in 1935 by description of seven patients with amenorrhea, hirsutism, and enlarged polycystic ovaries.
Because the diagnostic criteria for PCOS have changed, it is difficult to obtain consistent prevalence information. Approximately 22% of women in the general population have polycystic ovaries as an incidental finding on ultrasound examination. A study using the NIH/NICHHD criteria (which relied on oligomenorrhoea/amenorrhoea and clinical/biochemical evidence of hyperandroge-naemia) estimated that 6.2% of white and 3.4% of black women attending for a mandatory pre-employment medical examination had PCOS.2–8
Weight loss, exercise, and lifestyle modifications have been proven effective in restoring ovulatory cycles and achieving pregnancy in overweight women with PCOS and should be the first-line option for these women. Morbidly obese women should seek expert advise about pregnancy risk. Clomiphene citrate (CC) has been proven effective in ovulation induction for women with PCOS and should be considered the first-line therapy. Patients should be informed that there is an increased risk of multiple pregnancies with ovulation induction using CC. The CC will induce ovulation in 60–80% of properly selected patients. The likelihood of response decreases with increasing age and body mass index and the extent of associated hyperandrogenemia in anovulatory women. Women with amenorrhea are more likely to conceive than those with oligomenorrhea, possibly because infertile women who menstruate also likely ovulate infrequently and are more likely to have other co-existing infertility factors. Cumulative pregnancy rates of 70–75% can be expected over 6 cycles of treatment. Thereafter, cycle fecundability falls substantially. When pregnancy is not achieved within 3–6 of CC induced ovulatory cycles, the infertility investigation should be expanded to exclude other infertility factors, or to change the overall treatment strategy. CC resistance: It is defined as failure to ovulate in response to CC on doses of 150mg/day after 3 cycles. The CC resistance is an unpredictable and unpreventable event. It is virtually impossible to predict who will respond to which dose of CC. So far there is no general agreement on a standard regimen for management of CC resistant PCO patients.9
Several alternatives for those who are CC resistant: weight loss and lifestyle modifications: Obesity is strongly associated with PCOS and may be present in up to 50% of cases. Obese women with PCOS are more likely than thin women with PCOS to suffer from anovulation.10 This effect on ovulation may be secondary to insulin resistance, which in turn results in hyperinsulinemia and stimulation of excess androgen production from the ovaries.11 Lifestyle modification is the first line treatment in an evidence-based approach for the management of the majority of PCOS women who are overweight.12 Gonadotropins: ovulation induction for women with anovulatory PCOS using gonadotropin therapy is based on the physiological concept that initiation and maintenance of follicle growth may be achieved by a transient increase in follicle-stimulating hormone (FSH) above a threshold dose for sufficient duration to generate a limited number of developing follicles.12 Traditionally, ovulation induction with gonadotropins has been used as a second line treatment for CC resistant PCOS women; however, it is expensive, requires extensive monitoring and associated with significantly increased risk for ovarian hyperstimulation syndrome (OHSS) and multiple pregnancy.13
Laparoscopic ovarian diathermy (LOD): LOD is currently accepted as a successful second line treatment for ovulation induction in CC-resistant PCOS being as effective as gonadotropin treatment and is not associated with an increased risk of multiple pregnancy or OHSS.13 Insulin-sensitizing drugs: approximately 50–70% of all women with PCOS have some degree of insulin resistance.14 Hyperinsulinemia probably contributes to the hyperandrogenism which is responsible for the signs and symptoms of PCOS.15 Metformin, a biguanide, is now the most widely insulin sensitizer used for ovulation induction in women with PCOS. Third-generation aromatase inhibitors (AIs): third-generation AIs (anastrozole, letrozole, exemestane) are approved adjuvants for treatment of estrogen-receptor – positive breast cancer16 that were first used in ovulation induction in anovulatory women in 2001. Evidence suggests that nonsteroidal AIs, specifically letrozole and anastrozole, have ovulation-inducing effects by inhibiting androgen-to-estrogen conversion. Centrally, this effect releases the hypothalamic/pituitary axis from estrogenic negative feedback, increases gonadotropin secretion, and results in stimulation of ovarian follicle maturity. Moreover, peripherally, AIs may increase follicular sensitivity to FSH. Oral contraceptives: Branigan and Estes in a randomized controlled trial (RCT) showed that the suppression of the hypothalamic pituitary-ovarian axis for 2 months with combined oral contraceptives (COC) (0.03mg of ethinyl estradiol and 0.15mg of desogestrel) followed by CC, at dosage of 100mg/day on days 5–9 of the cycle, improved ovulation and pregnancy rates in CC resistant women in comparison with repeated cycles of CC alone. N-acetyl-cysteine (NAC): NAC is a mucolytic drug. Fulghesu et al. demonstrated that long term NAC treatment (1.8g/day for 5–6 weeks) was associated with significant increase in insulin sensitivity and reduction in insulin levels, testosterone and free androgen index (FAI) in hyperinsulinemic PCOS. Rizk et al. showed that the combination of NAC (1.2g/day) with CC (100mg/day) for only 5 days significantly increased both ovulation and pregnancy rates in obese women with CC-resistant PCOS compared with placebo (49.3% vs. 1.3% and 21.3% vs. 0, respectively). Dexamethazone therapy: dexamethazone therapy during the follicular phase has been described without any side effects or serious events. Parsanezhad et al. in a double-blind RCT, showed the safety and the efficacy of a high-dose short course of dexamethazone for inducing ovulation in 230 CC-resistant patients with PCOS and normal DHEAS levels. They reported significantly higher ovulation and pregnancy rates in those who received 200mg of CC (days 5–9) and 2mg of dexamethazone (days 5–14) compared with CC alone (88% vs. 20% and 40.5 vs. 4.2%, respectively). In these patients, dexamethazone reduced circulating dehydroepiandrosterone sulfate (DHEAS), total and free testosterone (T), and luteinizing hormone (LH) levels and the LH/FSH ratio after 2 weeks of treatment. These results were further confirmed in another RCT. Bromocriptine: currently, evidence suggests that PCOS and hyperprolactinaemia are two distinct entities without a pathophysiological link. Bromocriptine administration provided no benefit in CC-resistant PCOS patients with normal prolactin levels, receiving 150mg CC (days 5–9) and bromocriptin continuously administrated at a dosage of 7.5mg daily. In vitro fertilization: should be reserved for women with PCOS who fail other modalities or who have other indications for IVF treatment.14–17
The aim of the present study was to compare the efficacy of two different regimens of CC in the treatment of patients with PCOS resistant to the 150mg dose of CC.
PatientsThe study was conducted on 85 PCOS patients attended El-Shatby University Maternity Hospital in the period from 4/2012 to 9/2013 with the following inclusion criteria: PCOS diagnosis according to the Rotterdam criteria,3 age from 19 to 35 years old and no ovulation following a regimen of CC 100mg for 5 days (starting from day 3). Exclusion criteria: other causes of infertility than PCOS and other endocrinal disorders.
Patients were allocated to one of the following two groups: Group I: including 41 PCOS patients who received 200mg CC tablets for 7 days starting from day 3. Group II: including 44 PCOS patients who received 200mg CC and 2mg dexamethazone/daily for 10 days starting from day 3. The study was approved by the University Ethics Committee and a signed written informed consent was obtained from each patient.
MethodsFollowing approval of the Medical Ethical Committee, obtaining a signed informed written consent and explanation of the procedures and aim of the study, all women included in this study were subjected to: complete history taking, general medical examination; including a systematic endocrinal review, local gynecological examination, transvaginal ultrasound examination in order to evaluate ovarian volume, antral follicles count, uterus and endometrial thickness and pattern. Hormonal assays on day 2: FSH and LH levels, T levels and thyroid-stimulating hormone (TSH) and prolactin levels.
Follow-up: by folliculometry starting from day 11 then day after day for 1 week, endometrial thickness, mid luteal serum progesterone and chemical pregnancy.
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. Qualitative data were described using number and percent. Quantitative data were described using mean and standard deviation, median, minimum and maximum. Comparison between different groups regarding categorical variables was tested using Chi-square test. When more than 20% of the cells have expected count less than 5, correction for chi-square was conducted using Fisher's exact test or Monte Carlo correction. The distributions of quantitative variables were tested for normality using Kolmogorov–Smirnov test, Shapiro–Wilk test and D’Agstino test. For normally distributed data, comparison between different groups was analyzed using F-test (ANOVA). For abnormally distributed data, Kruskal Wallis test was used to compare between different groups. Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level.
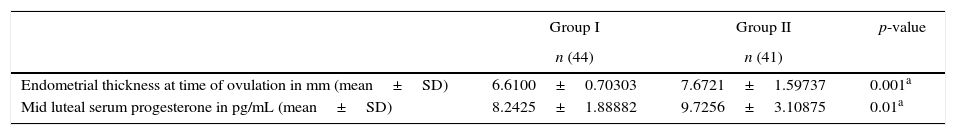
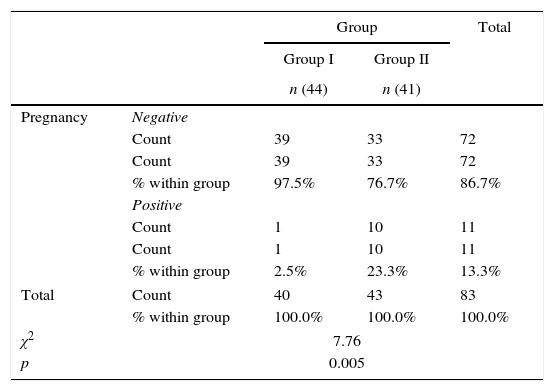
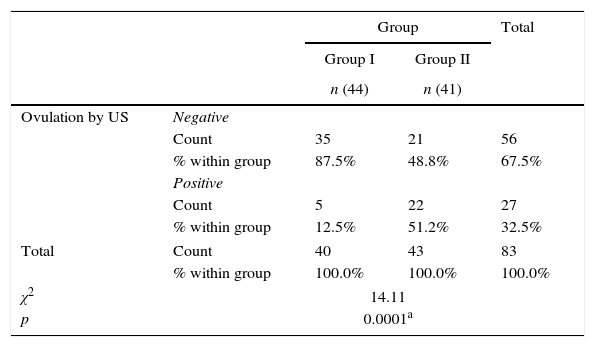
ResultsThere was no statistically significant difference between the groups regarding age, gravidity, parity, abortion, duration of marriage and weight (Table 1). There was statistical difference between both groups with larger endometrial thickness (7.67 vs. 6.61mm) and mid luteal serum progesterone (9.7 vs. 8.2ng/mL) in Group II (Table 2). There was significant difference between both groups with higher pregnancy rate (23.3% vs. 2.5%) in Group II (Table 3). There was significant difference between both groups with higher ovulation rate (51.2% vs. 12.5%) in Group II (Table 4).
Comparison between the two studied groups regarding demographic.
| Group I | Group II | p-Value | |
|---|---|---|---|
| n (44) | n (41) | ||
| Age in years (mean±SD) | 27.75±4.32 | 26.71±4.03 | 0.26 |
| Gravidity (mean±SD) | 0.48±0.68 | 0.49±0.88 | 0.939 |
| Parity (mean±SD) | 0.13±0.40 | 0.21±0.47 | 0.383 |
| Abortion (mean±SD) | 0.3750±0.627 | 0.302±0.741 | 0.632 |
| Duration of marriage in years (mean±SD) | 4.56±2.468 | 4.14±2.154 | 0.416 |
| Weight in kg (mean±SD) | 72.32±10.36 | 72.86±12.86 | 0.816 |
Pregnancy rate in the two studied groups.
| Group | Total | |||
|---|---|---|---|---|
| Group I | Group II | |||
| n (44) | n (41) | |||
| Pregnancy | Negative | |||
| Count | 39 | 33 | 72 | |
| Count | 39 | 33 | 72 | |
| % within group | 97.5% | 76.7% | 86.7% | |
| Positive | ||||
| Count | 1 | 10 | 11 | |
| Count | 1 | 10 | 11 | |
| % within group | 2.5% | 23.3% | 13.3% | |
| Total | Count | 40 | 43 | 83 |
| % within group | 100.0% | 100.0% | 100.0% | |
| χ2 | 7.76 | |||
| p | 0.005 | |||
Ovulation in the two studied groups.
| Group | Total | |||
|---|---|---|---|---|
| Group I | Group II | |||
| n (44) | n (41) | |||
| Ovulation by US | Negative | |||
| Count | 35 | 21 | 56 | |
| % within group | 87.5% | 48.8% | 67.5% | |
| Positive | ||||
| Count | 5 | 22 | 27 | |
| % within group | 12.5% | 51.2% | 32.5% | |
| Total | Count | 40 | 43 | 83 |
| % within group | 100.0% | 100.0% | 100.0% | |
| χ2 | 14.11 | |||
| p | 0.0001a | |||
p-Value for F-test (ANOVA) for comparing between the different studied group.
In 2002, the novel use of dexamethazone (high dose, short course) for inducing ovulation in anovulatory women with PCOS and normal DHEAS was reported. Dexamethazone was very well tolerated as no patients complained of any side effect. Dexamethazone therapy during the follicular phase has been described without any side effects or serious sequelae (Tables 1–4). It was found that there were no differences in ovulatory response to dexamethazone therapy between women with or without DHEAS excess. On the other hand, in 1984, it was reported that the effect of dexamethazone was largest in women with raised DHEAS levels.10–13
The recent systematic review of the Cochrane library reported that the use of dexamethazone as an adjunct to CC appears promising improvement in the pregnancy rate when compared to CC alone.
In our study, we used comparison between 2 different regimens for management of PCOS resistant to 150mg CC. The first group included 44 females received 200mg CC in and the other with addition of 2mg dexamethazone daily for 10 days starting from day 3. Then follow up was done by TVUS, mid luteal serum progesterone and serum pregnancy test. We found that a significant higher rate of ovulation was reported in the dexamethazone group compared to CC group. As regard to endometrial thickness was more favorable (in dexamethazone group than CC only. As regard to mid luteal serum progesterone it was found higher level in dexamesathone group (compared to CC only. As regard to serum pregnancy it was higher in dexamethazone group than CC only. Our result is in agreement with Parsanezhad et al.’s, Elnashar et al.’s and Al-Shaikh's study. Parsanezhad et al.’s study was done in 2002 at Shiraz University of Medical Sciences, Shiraz, Iran on 230 PCOS females resistant to 250mg CC divided randomly to two groups. First group (control group) received 200mg of CC from cycle days 5 to 9 and four tablets of placebo (folic acid) from cycle days 5 to 14. Second group received 200mg of CC from cycle days 5 to 9 and 2mg of dexamethazone tablets from cycle days 5 to 14. The result of this study confirmed that high-dose dexamethazone during the follicular phase resulted in a high rate of ovulation with a pregnancy rate of 40% with sharp contrast to the low ovulation and pregnancy rates seen in the other group. Elnashar et al.’s study done in 2006 at the Department of Obstetrics and Gynecology, Benha University Hospital, Benha, Egypt on 80 PCOS females resistant to CC divided randomly to two groups. First group received CC 100mg/day from day 3 to day 7 of the cycle and dexamethazone 2mg/day from day 3 to day 12 of the cycle. Second group received CC 100mg/day from day 3 to day 7 of the cycle and placebo (folic acid tablets) from day 3 to day 12 of the cycle. In this study, a significant higher rate of ovulation was reported in the dexamethazone group (75%) than in placebo group (15%). Similarly the mean number of follicles >18mm at the time of human chorionic gonadotropin (hCG) administration was significantly higher in the dexamethazone group than in the placebo group, the mean endometrial thickness and the pregnancy rate in the dexamethazone group (8.8mm) was significantly higher than in the placebo group (7mm) and a higher rate of pregnancy in dexamethazone group (40%) than in placebo group (5%).12–14
Al-Shaikh's study was done at Infertility Clinic of Babil Hospital between April 2008 and October 2009 on 43 PCOS females resistant to 100mg CC. First group (31 patients) received CC 100mg/day from day 3 to day 7 of the cycle and prednisolone 10mg/day orally in two divided doses from day 2 to day 12 of the cycle. Second group (12 patients) received CC 150mg/day from day 3 to day 7 of the cycle. In this study, a significant higher rate of ovulation was reported in the prednisolone group 23 (74.19%) versus 2 (16.66%) in CC only group and in the clomiphene plus prednisolone treatment group there is a significant improvement in the pregnancy rate when compared to CC alone 17 (54.84%) versus 1 (8.33%) respectively (Tables 1–4). There are a number of potential mechanisms by which dexamethazone (corticosteroids) may affect ovarian function. Dexamethazone acts directly on the pituitary gland to suppress the action of estradiol, which may be involved in the process of induction of ovulation by glucocorticoid–clomiphene treatment. Dexamethazone may directly influence follicular development. Dexamethazone may act indirectly by increasing serum GH, serum insulin-like growth factor (IGF-1) and consequently follicular fluid IGF-1 concentrations. Dexamethazone enhances the FSH-stimulated follicular steroid production. The synergism of Dexamethazone (corticosteroids) with FSH in follicular progesterone production and its inhibition by transforming growth factor-β (TGF-β) indicate that glucocorticoid and TGF-β interaction may be necessary for granulosa cell differentiation as follicle matures through pre-antral stages.18 Casterlin et al. found that the majority of pregnancies (97%) occurred when the endometrial thickness was 7–11mm, and the miscarriage rate doubles when the endometrial thickness measures <7mm. Immunosuppression, leading to a favorable endometrial environment, was the rationale behind the administration of high-dose glucocorticoid from embryo transfer onwards, and higher implantation rates have been observed.19 Although the mechanism underlying the beneficial effects of dexamethazone (corticosteroids) is not exactly understood, it is apparent from our data that dexamethazone (corticosteroids) therapy during the follicular phase can enhance follicular development and ovulation.12–16
ConclusionsDexamethazone (corticosteroids) therapy combined with CC can improve folliculogenesis, ovulation, and pregnancy rate in CC resistant PCOS. Using CC alone by dose of 200mg for 7 days in PCOS patients resistant to 150mg CC showed no aberrant difference in ovulation rate and pregnancy rate. It is apparent from our data that dexamethazone (corticosteroids) therapy during the follicular phase can enhance follicular development and ovulation. So we recommend use of dexamethazone (corticosteroids) before shift to expensive gonadotropins in PCOS females resistant to CC.
Conflicts of interestThe authors declare no conflicts of interest.