Preoperative localization of parathyroid pathology, generally a parathyroid adenoma, can be difficult in some cases due to the anatomical variants that these glands present. The objective of this review is to analyse the different imaging techniques used for preoperative localization of parathyroid pathology (scintigraphy, ultrasound, CT, MRI and PET).
There is great variability between the different tests for the preoperative localization of parathyroid pathology. The importance of knowing the different diagnostic options lies in the need to choose the most suitable test at each moment and for each patient for an adequate management of primary hyperparathyroidism (PHP) with surgical criteria.
La localización preoperatoria de patología paratiroidea, por lo general un adenoma paratiroideo, puede ser difícil en algunos casos debido a las variantes anatómicas que presentan estas glándulas. El objetivo de esta revisión es analizar las diferentes técnicas de diagnóstico por imagen utilizadas en la localización preoperatoria de patología paratiroidea (gammagrafía, ecografía, TC, RM y PET).
Existe una gran variabilidad entre las diferentes pruebas para la localización preoperatoria de patología paratiroidea. La importancia de conocer las diferentes opciones de diagnóstico radica en la necesidad de elegir la técnica más adecuada en cada ocasión y para cada paciente para un manejo adecuado del hiperparatiroidismo primario (HPTP) con criterio quirúrgico.
Parathyroid glands are small oval structures located in two on each side of the posterior aspect of the thyroid gland, weighing less than 50 mg each, and measuring about 6 × 3−4 mm in diameter (craniocaudal × transverse).1 These glands are made up of three types of cells, 50%–60% chief cells (which contain few mitochondria and secrete parathyroid hormone (PTH)), 30%–40% clear cells (which become chief cells with age, contain a lot of cytoplasmic glycogen and whose real function is unknown) and less than 5% of oxyphilic cells (rich in mitochondria and whose function is also unknown).2
Primary hyperparathyroidism (PHP) is the third most common endocrine disorder after diabetes and thyroid disease, and is the most common cause of hypercalcaemia, secondary to excessive PTH secretion. It affects 0.3%–1% of the population, being more frequent in women with a 3:1 ratio compared to men, with a maximum incidence in the fourth and fifth decades of life.1,3–5
Nowadays, the most frequent presentation is asymptomatic. In cases of symptomatic PHP, the organs most commonly affected are bone, due to decreased bone mineral density and its consequent fractures, followed by the kidney, due to the damage caused by nephrolithiasis. There are other less frequent symptoms, among which we find gastrointestinal problems (pancreatitis, peptic ulcer, constipation, nausea, vomiting or gastroesophageal reflux), cardiovascular problems (hypertension, calcifications of the heart valves and myocardium, hypertrophy of the left ventricle and shortening of the QT segment, which favours arrhythmias and increased mortality from acute myocardial infarction and stroke) and neuropsychiatric conditions (confusion, depression, cognitive impairment, sleep disorders, irritability or decreased concentration).1,6
Regarding its aetiology, up to 85%–90% of PHP cases are due to the presence of a solitary parathyroid adenoma, 5%–10% due to hyperplasia of the gland and <1% to parathyroid carcinoma.1
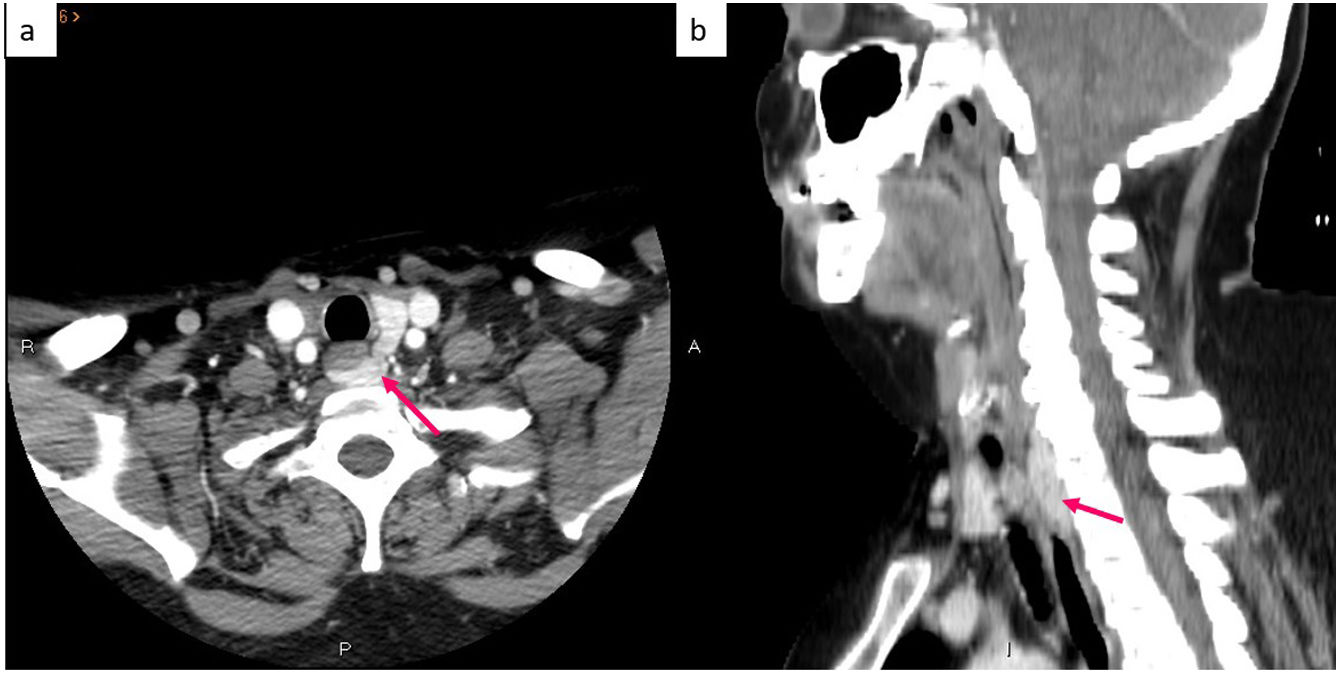
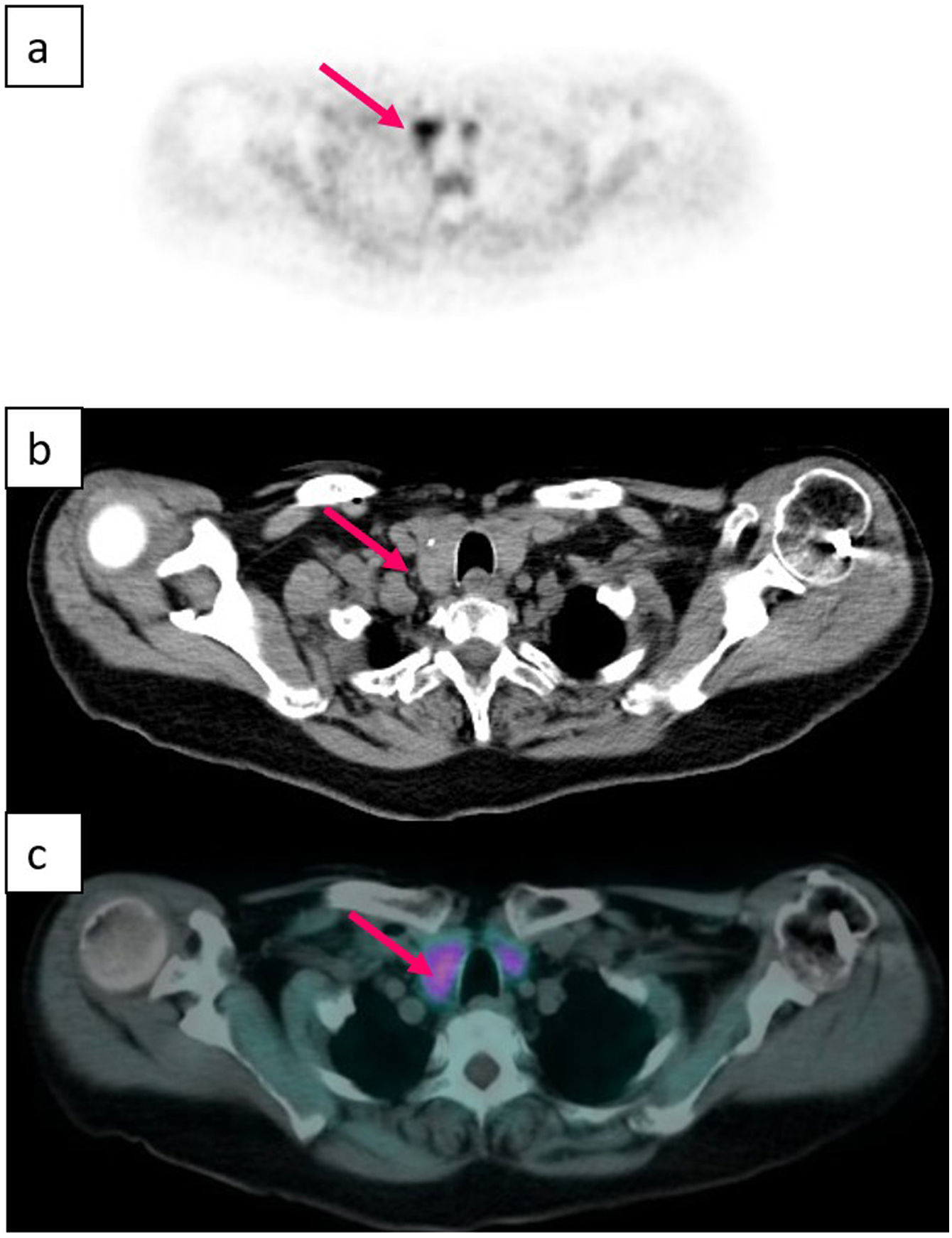
The diagnosis of PHP is made by means of an adequate clinical history together with a measurement of calcium corrected by albumin and PTH in serum, and the only effective treatment is surgery. Classically, surgery consisted of a bilateral exploration of the neck and excision of the affected glands,4 but the fact that the majority of PHP cases are due to disease of a single gland, together with the improvement of intraoperative monitoring of PTH and preoperative localization using imaging studies have favoured the development of minimally invasive parathyroidectomy.2,7 This latter modality has similar cure rates to bilateral surgical exploration, with similar recurrence, persistence, and reoperation rates. However, by minimising tissue dissection, it speeds up recovery time, reduces postoperative pain, and decreases scarring (and therefore leads to better aesthetic results), as well as having lower cost and less complications. In addition, bilateral surgical exploration requires hospital admission, while unilateral surgery is an outpatient procedure, thus it has been established as a safe and effective method.8–11 The success of minimally invasive surgery undoubtedly depends on the experience of the surgeon,1 but also on the precise pre-surgical localisation of the affected parathyroid gland, including not only lateralization but also the quadrant specifically.12 However, locating the pathological parathyroid by means of imaging techniques is frequently problematic due to the anatomical variants that these glands can present, especially the inferior ones due to their more prolonged embryological migration.8 In fact, approximately 16% of cases have one or more hyperfunctioning parathyroids in an ectopic location3 (Figs. 1–6).
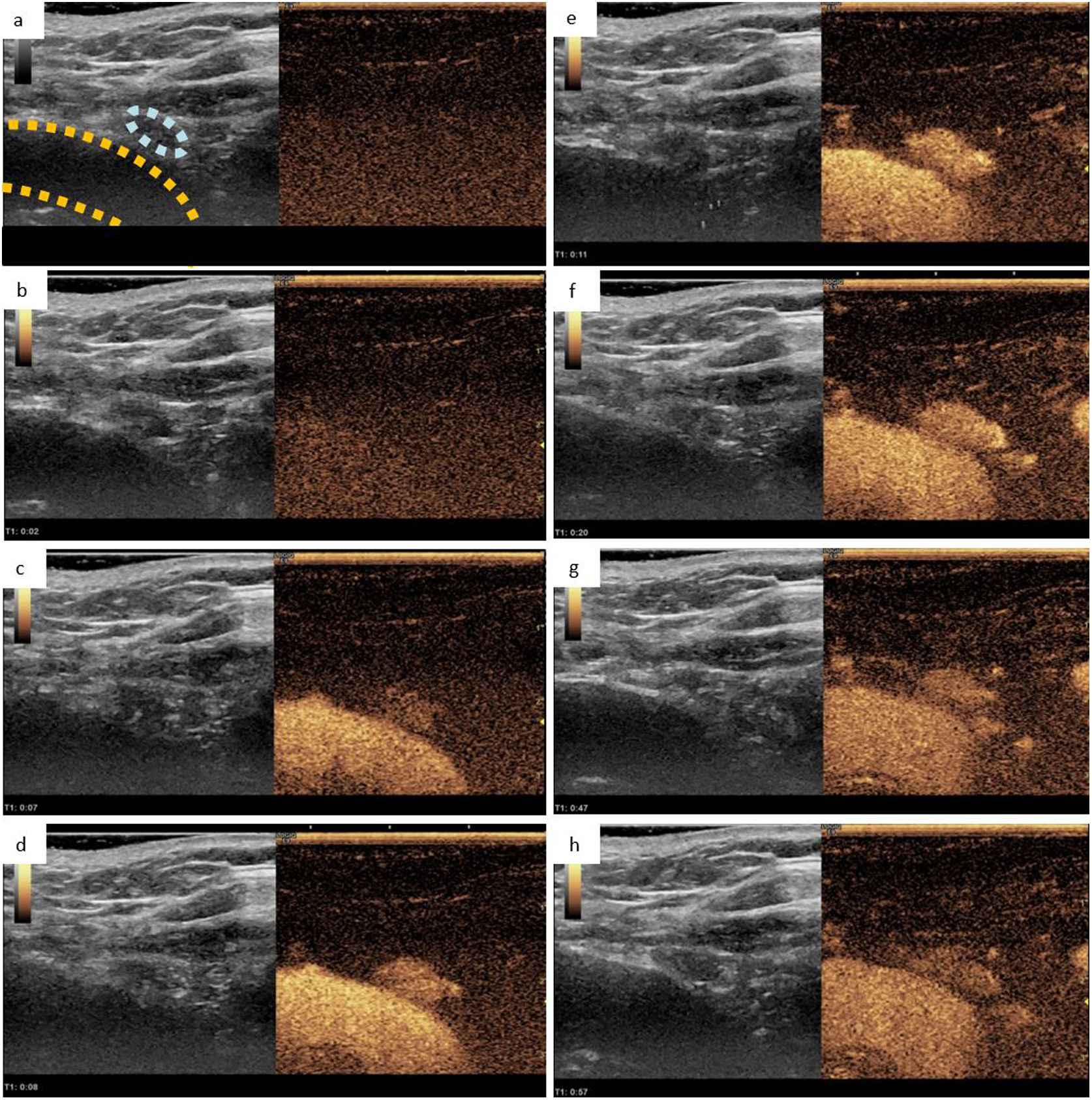
Right inferior parathyroid adenoma observed in CEUS. a) B-mode ultrasound showing a 5 mm hypoechoic lesion (circled light blue) adjacent to the right brachiocephalic trunk (circled yellow), coupled with an image exhibiting a lower mechanical index that allows for the visualisation of the contrast medium, both obtained before its administration. B-h) Representation of the dynamic study at 2, 7, 8, 11, 20, 47 and 57 s respectively after the administration of ultrasound contrast. In this dynamic study, we can observe how the suspicious lesion presents an intense and early enhancement with delayed lavage, findings suggestive of parathyroid adenoma. Obtained from a patient at our hospital.
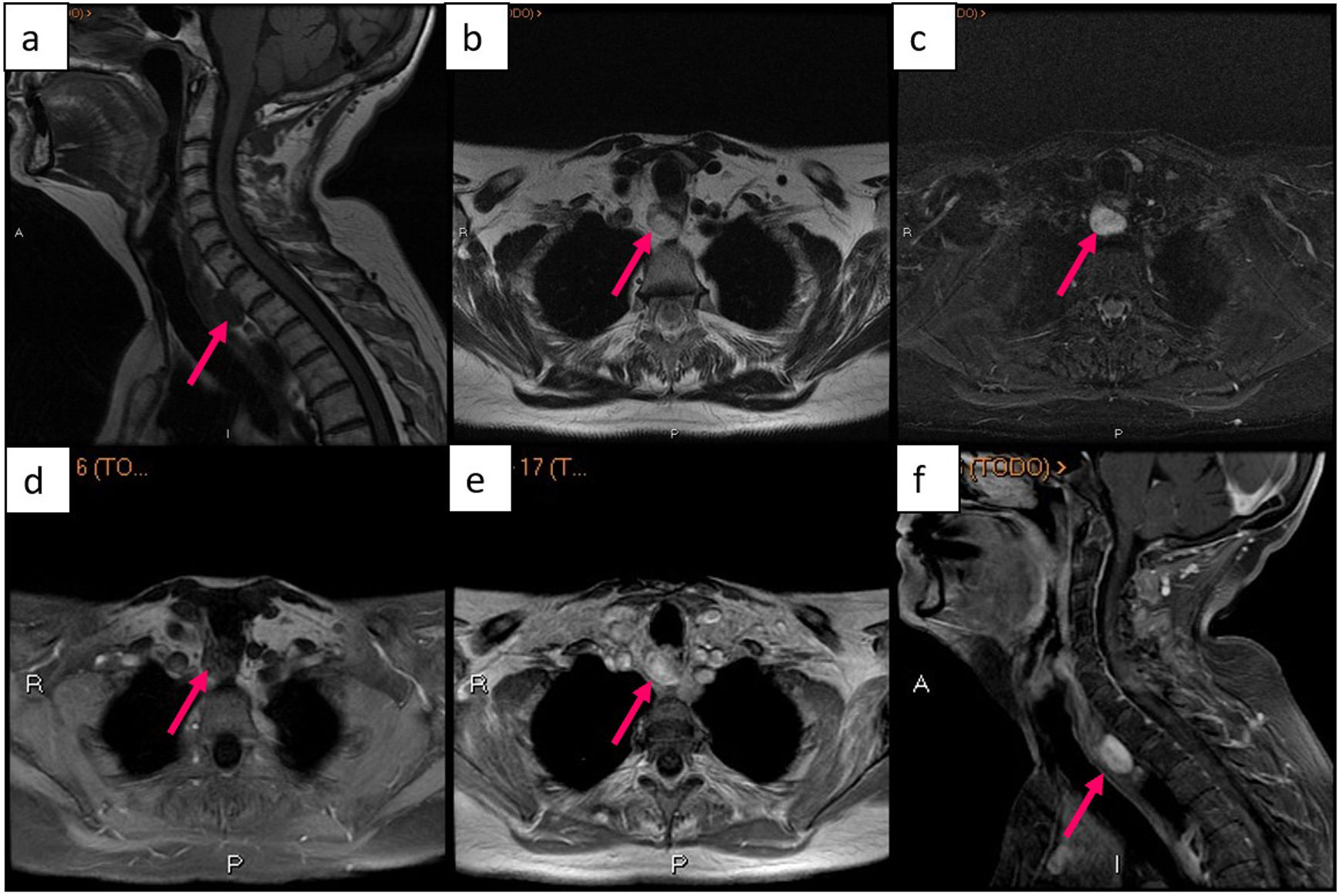
Retrotracheal parathyroid adenoma seen on MRI. (a) sagittal image of T1-weighted sequence, (b) axial image of T2-weighted sequence, (c) axial image of STIR sequence, (d) axial image of T1-weighted sequence with fat suppression and without contrast, (e) axial image of T1-weighted sequence with contrast, (f) sagittal image of T1-weighted sequence with fat suppression and contrast. Obtained from a patient at our hospital.
This article has two main objectives: to update the state of the art of the role of different imaging techniques used for preoperative localization of parathyroid pathology and to expose the different advantages, disadvantages and causes of false positives and negatives of the different techniques.
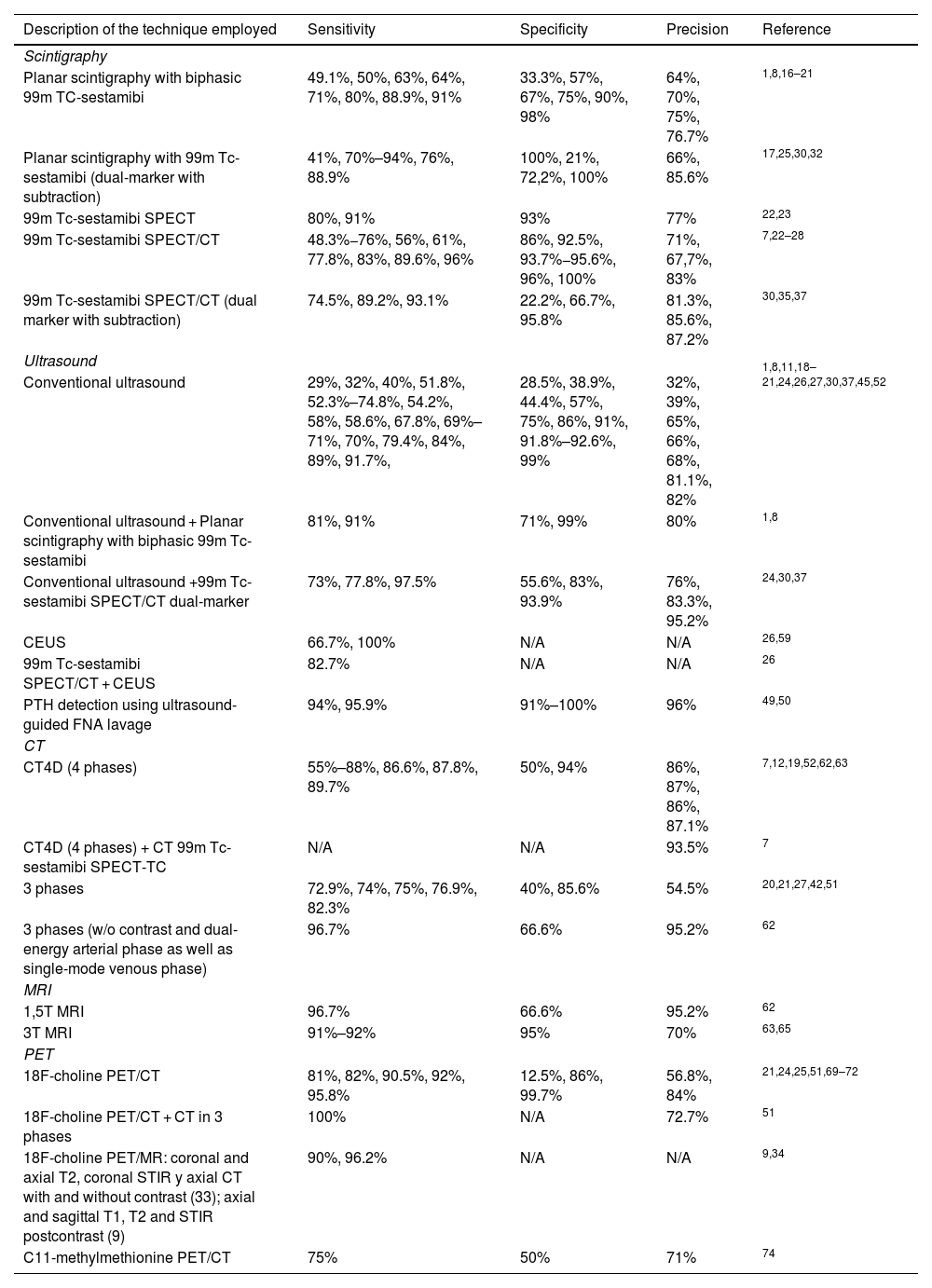
Overview of the different imaging techniquesThe imaging tests used for the preoperative localization of parathyroid pathology that are included in the reviewed articles are scintigraphy, cervical ultrasound, computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET). Each is discussed in detail below, describing the sensitivity, specificity, and diagnostic accuracy of the different tests in Table 1.
Sensitivity, specificity and precision of different imaging tests used for the localization of parathyroid pathology. N/A (not available).
| Description of the technique employed | Sensitivity | Specificity | Precision | Reference |
|---|---|---|---|---|
| Scintigraphy | ||||
| Planar scintigraphy with biphasic 99m TC-sestamibi | 49.1%, 50%, 63%, 64%, 71%, 80%, 88.9%, 91% | 33.3%, 57%, 67%, 75%, 90%, 98% | 64%, 70%, 75%, 76.7% | 1,8,16–21 |
| Planar scintigraphy with 99m Tc-sestamibi (dual-marker with subtraction) | 41%, 70%–94%, 76%, 88.9% | 100%, 21%, 72,2%, 100% | 66%, 85.6% | 17,25,30,32 |
| 99m Tc-sestamibi SPECT | 80%, 91% | 93% | 77% | 22,23 |
| 99m Tc-sestamibi SPECT/CT | 48.3%−76%, 56%, 61%, 77.8%, 83%, 89.6%, 96% | 86%, 92.5%, 93.7%−95.6%, 96%, 100% | 71%, 67,7%, 83% | 7,22–28 |
| 99m Tc-sestamibi SPECT/CT (dual marker with subtraction) | 74.5%, 89.2%, 93.1% | 22.2%, 66.7%, 95.8% | 81.3%, 85.6%, 87.2% | 30,35,37 |
| Ultrasound | ||||
| Conventional ultrasound | 29%, 32%, 40%, 51.8%, 52.3%–74.8%, 54.2%, 58%, 58.6%, 67.8%, 69%–71%, 70%, 79.4%, 84%, 89%, 91.7%, | 28.5%, 38.9%, 44.4%, 57%, 75%, 86%, 91%, 91.8%–92.6%, 99% | 32%, 39%, 65%, 66%, 68%, 81.1%, 82% | 1,8,11,18–21,24,26,27,30,37,45,52 |
| Conventional ultrasound + Planar scintigraphy with biphasic 99m Tc-sestamibi | 81%, 91% | 71%, 99% | 80% | 1,8 |
| Conventional ultrasound +99m Tc-sestamibi SPECT/CT dual-marker | 73%, 77.8%, 97.5% | 55.6%, 83%, 93.9% | 76%, 83.3%, 95.2% | 24,30,37 |
| CEUS | 66.7%, 100% | N/A | N/A | 26,59 |
| 99m Tc-sestamibi SPECT/CT + CEUS | 82.7% | N/A | N/A | 26 |
| PTH detection using ultrasound-guided FNA lavage | 94%, 95.9% | 91%–100% | 96% | 49,50 |
| CT | ||||
| CT4D (4 phases) | 55%–88%, 86.6%, 87.8%, 89.7% | 50%, 94% | 86%, 87%, 86%, 87.1% | 7,12,19,52,62,63 |
| CT4D (4 phases) + CT 99m Tc-sestamibi SPECT-TC | N/A | N/A | 93.5% | 7 |
| 3 phases | 72.9%, 74%, 75%, 76.9%, 82.3% | 40%, 85.6% | 54.5% | 20,21,27,42,51 |
| 3 phases (w/o contrast and dual-energy arterial phase as well as single-mode venous phase) | 96.7% | 66.6% | 95.2% | 62 |
| MRI | ||||
| 1,5T MRI | 96.7% | 66.6% | 95.2% | 62 |
| 3T MRI | 91%–92% | 95% | 70% | 63,65 |
| PET | ||||
| 18F-choline PET/CT | 81%, 82%, 90.5%, 92%, 95.8% | 12.5%, 86%, 99.7% | 56.8%, 84% | 21,24,25,51,69–72 |
| 18F-choline PET/CT + CT in 3 phases | 100% | N/A | 72.7% | 51 |
| 18F-choline PET/MR: coronal and axial T2, coronal STIR y axial CT with and without contrast (33); axial and sagittal T1, T2 and STIR postcontrast (9) | 90%, 96.2% | N/A | N/A | 9,34 |
| C11-methylmethionine PET/CT | 75% | 50% | 71% | 74 |
CT: computed tomography; MRI: magnetic resonance imaging; SPECT/CT: single photon emission computed tomography/computed tomography; STIR: Short I Inversion Recovery.
In 1989, Coakley et al. fortuitously discovered that the 99m Tc-sestamibi (sesta-methoxyisobutylisonitrile) used for myocardial perfusion testing was predominantly taken up by abnormal parathyroid glands.13 Sestamibi is a lipophilic cation that passively crosses cell membranes and is trapped in mitochondria due to the negative transmembrane potential. The exact mechanism of its uptake by the parathyroid glands is not entirely clear. It is thought to be due to a combination of factors, including increased blood flow and increased presence of oxyphilic cells, which are rich in mitochondria, by the hyperfunctioning parathyroid glands.14,15 In this way, 99m Tc-sestamibi is taken up by both the thyroid and parathyroid glands, although there is a difference in wash-out between the two, so that the typical image of parathyroid adenoma consists of focal hyper-uptake in the early phase (5−20 min after its administration, according to different protocols), with a slow washout in the late phase (at 120−180 min). This double-phase technique is the most classically used.13–15
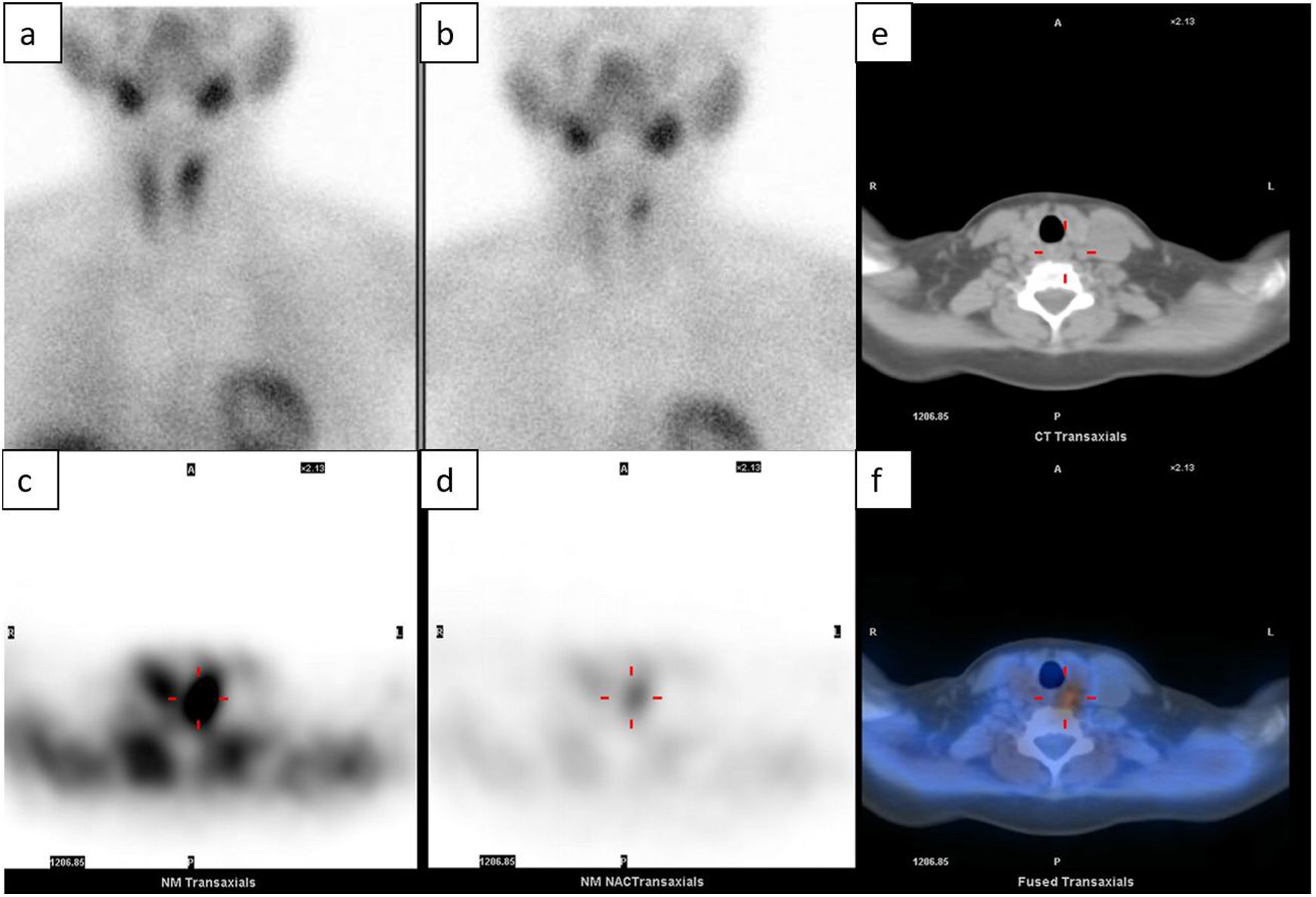
There are multiple studies published on planar scintigraphy used in this context, reporting a sensitivity of 49.1%–91%, a specificity of 33.3%–98%, and an accuracy of 64%–76.7% for the detection of parathyroid adenoma.1,8,16–21 For its part, SPECT (Single Photon Emission Computed Tomography) allows three-dimensional volumetric detection, improving the sensitivity of 99m Tc-sestamibi for the detection of a solitary adenoma before surgery up to 80%–91%, with a specificity of 93% and an accuracy of 77%.22,23 Likewise, 99m Tc-sestamibi SPECT in combination with computed tomography (CT) improves the detection of parathyroid adenomas, reaching a sensitivity of 48.3%–96%, a specificity of 86%–100% and an accuracy of 67.7%–83%7,22–28 (Table 1). In addition, this combination with CT allows for precise anatomical localization before surgery also for ectopic retrotracheal or mediastinal adenomas, facilitating adequate surgical planning.16
There is no consensus regarding the dose of 99m Tc-sestamibi that should be administered, which varies according to different studies between 10mCi (370 MBq)17 and 25mCi (925 MBq).7,9
Some authors state that delayed-phase imaging does not improve diagnostic accuracy, because residual parathyroid activity on delayed imaging is already present on early imaging, only obscured by background thyroid activity, and therefore quantitative interpretation on initial scans are sufficient for diagnosis.18
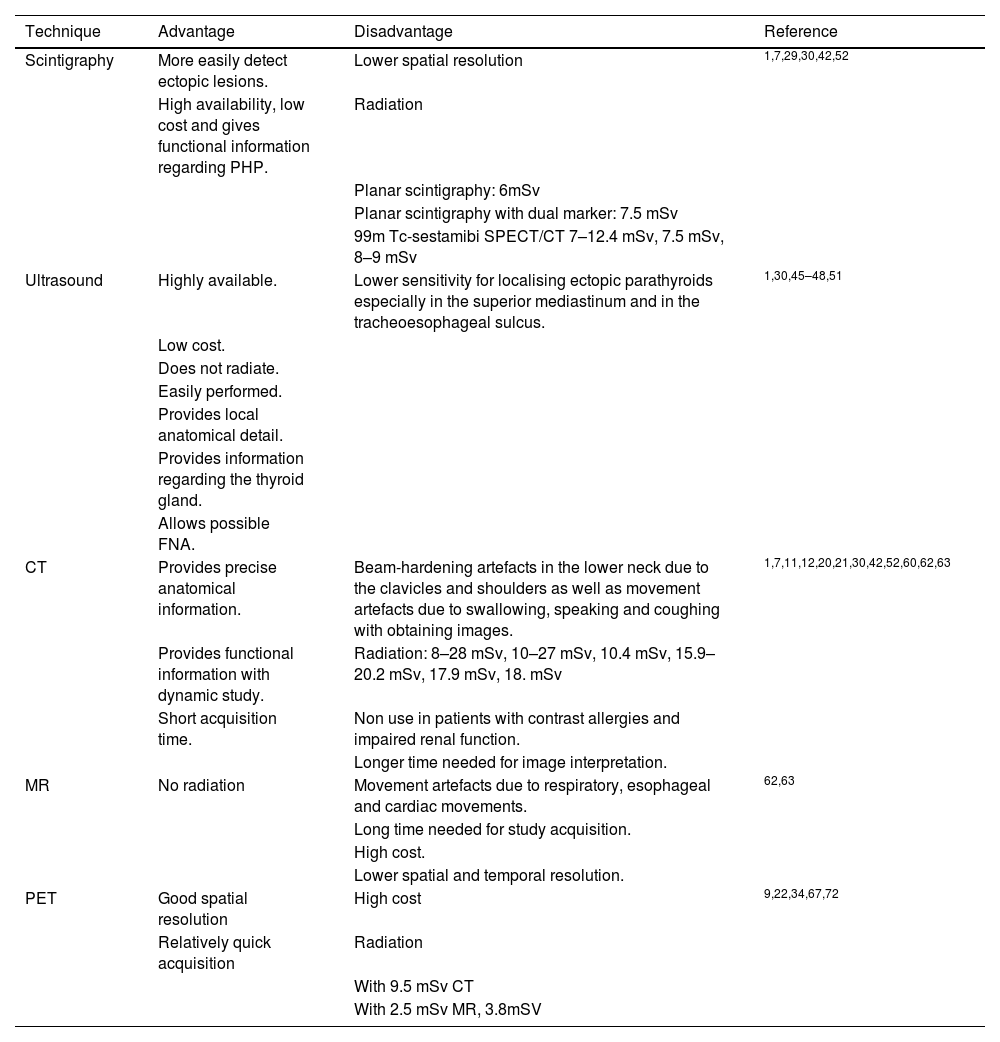
Among the advantages of this technique are that it is easy to perform, it is readily available, has a low cost, it allows ectopic lesions to be detected more easily and it provides functional information in relation to PHP.29,30 Its disadvantages include poor anatomical delimitation and lower spatial resolution31 (Table 2). Furthermore, the typical 99m Tc-sestamibi uptake and washout pattern occurs in 70%–75% of cases. In some patients, lavage of the parathyroid adenoma and the thyroid gland occur at the same time, which is called rapid lavage. It is believed to be related to factors such as the number of cells rich in mitochondria or the degree of angiogenesis.32 That is why there are double isotope modalities, so that this second isotope is captured only by the thyroid and the subtraction of images allows for the diagnosis of the parathyroid lesion. That second isotope used can be I12332–34 or Tc 99m-pertechnetate.17,35,36 The studies report different ranges of sensitivity, specificity, and localization accuracy (Table 1).
Advantages and disadvantages of the diagnostic techniques used.
| Technique | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Scintigraphy | More easily detect ectopic lesions. | Lower spatial resolution | 1,7,29,30,42,52 |
| High availability, low cost and gives functional information regarding PHP. | Radiation | ||
| Planar scintigraphy: 6mSv | |||
| Planar scintigraphy with dual marker: 7.5 mSv | |||
| 99m Tc-sestamibi SPECT/CT 7–12.4 mSv, 7.5 mSv, 8–9 mSv | |||
| Ultrasound | Highly available. | Lower sensitivity for localising ectopic parathyroids especially in the superior mediastinum and in the tracheoesophageal sulcus. | 1,30,45–48,51 |
| Low cost. | |||
| Does not radiate. | |||
| Easily performed. | |||
| Provides local anatomical detail. | |||
| Provides information regarding the thyroid gland. | |||
| Allows possible FNA. | |||
| CT | Provides precise anatomical information. | Beam-hardening artefacts in the lower neck due to the clavicles and shoulders as well as movement artefacts due to swallowing, speaking and coughing with obtaining images. | 1,7,11,12,20,21,30,42,52,60,62,63 |
| Provides functional information with dynamic study. | Radiation: 8–28 mSv, 10–27 mSv, 10.4 mSv, 15.9–20.2 mSv, 17.9 mSv, 18. mSv | ||
| Short acquisition time. | Non use in patients with contrast allergies and impaired renal function. | ||
| Longer time needed for image interpretation. | |||
| MR | No radiation | Movement artefacts due to respiratory, esophageal and cardiac movements. | 62,63 |
| Long time needed for study acquisition. | |||
| High cost. | |||
| Lower spatial and temporal resolution. | |||
| PET | Good spatial resolution | High cost | 9,22,34,67,72 |
| Relatively quick acquisition | Radiation | ||
| With 9.5 mSv CT | |||
| With 2.5 mSv MR, 3.8mSV |
CT: computed tomography; FNA: fine needle aspiration; MRI: magnetic resonance imaging; PET: positron emission tomography; PHP: primary hyperparathyroidism; SPECT/CT: single photon emission computed tomography/computed tomography.
Other causes of false negatives in scintigraphic studies are the small size of the adenomas, the presence of ectopic glands, multiglandular disease, right-sided or superiorly located adenomas, or taking NSAIDs.28,32,38–40 In addition, P-glycoprotein is a multidrug-resistant plasma membrane lipoprotein and it is believed that, in the same way that its overexpression increases the release of chemotherapeutic drugs from cancer cells, it also expels sestamibi from oxyphil cells, causing false negatives in scintigraphic studies.35,41
Another aspect of concern is exposure to ionizing radiation, which is estimated to have an effective dose of approximately 6 mSv in the case of techniques with using single tracer,1 7.5 mSv if double tracer with subtraction is used1 and 7−12.4 mSv if associated with low-dose CT1,7,42 (Table 2). Since a plain chest radiograph is approximately 0.02 mSv, these modalities would correspond to approximately 300, 375, and 400–500 chest radiographs, respectively.1,23
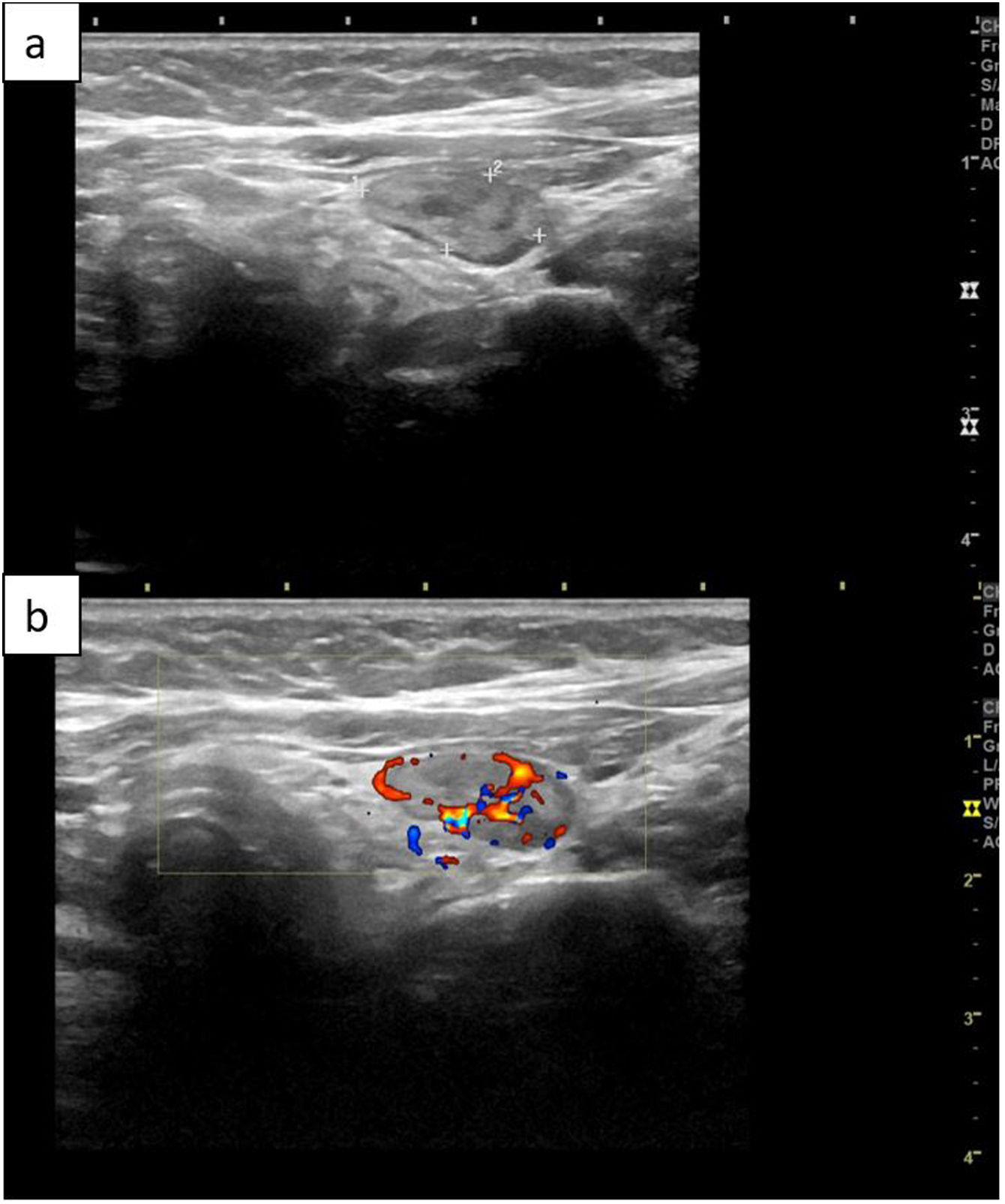
Cervical ultrasoundNormal parathyroid glands are not visible with this technique. A parathyroid adenoma is typically seen on B-mode as a well-defined and homogeneously hypoechoic lesion. On Doppler mode, they usually appear as hypervascular masses.5,43 When we see a compatible structure, it must be observed in two planes (longitudinal and transverse) to rule out that it is a false image, for example, due to the prevertebral musculature.43
In fact, lymph nodes frequently appear as hypoechoic rounded structures and can be confused with parathyroid adenomas. The presence of a hypervascular central fatty hilum in the Doppler mode indicates that it is a lymph node. However, reactive lymph nodes, typically associated with autoimmune thyroiditis, often do not show a hilum, and can be misleading.43
Besides, parathyroid carcinoma should be also included in the differential diagnosis, despite its low prevalence, which is reported around 1%. Although there are signs that make us suspect the existence of a carcinoma such as an enlarged, heterogeneous parathyroid gland with irregular borders, or with evidence of invasion of adjacent structures, as well as the lesion being taller than it is wide or having calcifications inside,43–45 Huang et al. found that there were no differences between parathyroid carcinoma and adenoma in terms of echogenicity, maximum diameter, internal cystic areas, age, gender distribution, or serum calcium levels. However, high levels of PTH do correlate with the existence of carcinoma, so that finding PTH levels above 1000 pg/mL associated with intralesional calcifications has a sensitivity of 71% and a specificity of 100% for a malignant tumour.45
The advantages of this technique are its high availability, absence of ionizing radiation and low cost.1,30,45 In addition, it allows for the identification of concomitant thyroid pathology1,30,46,47 and, when in doubt, an ultrasound-guided fine-needle aspiration allows the existence of malignant thyroid cells to be ruled out by cytology48 (Table 2). Likewise, an FNA wash can be done with 0.5–1 cc of 0.9% saline solution to quantify PTH levels, so that if the PTH levels in the wash are at least twice the PTH levels of a blood sample obtained simultaneously, we can conclude the presence of a parathyroid lesion with a sensitivity of 94%–95.9%, specificity of 91%−100% and precision of 96%49,50 (Table 1).
Disadvantages include the difficulty in evaluating the parathyroid glands in the superior mediastinum or retrotracheal region, lower sensitivity for detecting hyperplastic glands, as well as difficulties arising from the patient's condition (for example, having an obese neck or cervical hyperkyphosis)1,5,30,44,51 (Table 2).
There is much variability in the literature regarding the diagnostic sensitivity of ultrasound, which ranges between 29% and 91.7%, specificity between 28.5%–99%, and accuracy between 32%–82%,1,8,11,18–21,24,26,27,30,37,45,52 where clearly the most important factor influencing the sensitivity variability of this test is the experience of the personnel performing it1,43 (Table 1).
Another modality associated with this technique is contrast-enhanced ultrasound or CEUS (Contrast Enhanced UltraSound). Ultrasound contrast is made up of microbubbles of sulphur hexafluoride, an inert gas, coated by phospholipids.53,54 When these microbubbles are subjected to an ultrasound beam, a reflection of the wave is produced on the surface of the bubble due to the large difference in acoustic impedance between the gas and the plasma, which translates into a hyperechoic ultrasound signal that the sonographer interprets.55 It is an extremely safe contrast, with a low incidence of adverse effects, without cardiotoxic, hepatotoxic or nephrotoxic effects.56–58
Among its disadvantages are the following; an intravenous line must be placed for its administration and it requires two people, one to perform the ultrasound and the other to inject the contrast. Furthermore, it does not allow evaluation of more than one or two specific lesions, although this fact can be corrected by administering subsequent doses of contrast.53,58 It has been used mainly for the characterization of focal liver lesions31,54 and its use for parathyroid localization is not yet listed in the data sheet. However, some articles point out that it can be a promising and profitable technique for locating parathyroid pathology with a diagnostic sensitivity higher than ultrasound, and can help differentiate parathyroid adenomas from lymph nodes and thyroid lesions based on the pattern of contrast uptake. Findings point to a parathyroid lesion if we observe early arterial hypervascularization in the first 30 s from the periphery towards the centre, followed by central lavage in the late phase up to 120 s. In the event that the suspicious lesion shows progressive centripetal enhancement in the late phase, it will indicate that we are dealing with adenopathy or thyroid tissue.26,59 Parra Ramirez et al. informed a diagnostic sensitivity of CEUS of 66.7%, and in combination with 99m Tc-sestamibi SPECT/CT of 82.7%.26 However, Platz et al. reported a diagnostic sensitivity of CEUS of 100%59 (Table 1).
Computed tomographyAnother technique that has been investigated in recent years is the use of multiphasic CT, initially described as having 4 phases (hence the name 4dCT), performing a phase without contrast, followed by the injection of 60−120 mL of non-ionic iodinated contrast at a speed of 4−5 mL/s, obtaining images from the angle of the mandible to the carina in the arterial phase at 15–25 s, in the early venous phase at 55–65 s and in the late venous phase or delayed phase at 85–100 s.7,12,60
In the arterial phase, parathyroid lesions present a significant enhance reaching 138–180 HU. The maximum uptake has been described to be between 25–60 s after injection. This fact makes it possible to differentiate these lesions from lymph nodes, which show a progressively increasing enhancement after contrast injection, being maximum in late phases. Likewise, uptake characteristics also makes it possible to differentiate parathyroid lesions from thyroid nodules which, although they also enhance intensely in the arterial phase, couldhave high attenuation values in the non-contrast phase due to their high iodine content. In this way, parathyroid adenomas can be diagnosed with a sensitivity that varies from 55% to 89.7%, specificity between 50%–94% and diagnostic accuracy between 86%–87.1% according to different studies7,12,19,52,61,62 (Table 1).
The main drawback of CT is the high amount of radiation received by the patient, with the average effective dose by multiphase CT being 10–28 mSv according to different authors7,11,12,21,42,52,60,62 (Table 2). This concern has led to the performance of studies in three or even two phases, with equivalent diagnostic accuracy but less radiation,20,21,27,42,51,60 or even to the use of newer CT scans. Dual Energy using different kilovoltages, being able to reduce radiation to 3–5 mSv (lower than 99m Tc-sestamibi SPECT/CT)11 (Table 1). Moosvi et al. affirm that, despite the fact that the overall risk of developing neoplasia may increase (the average radiation absorbed by a person in the United Kingdom is 2.7 mSv/year), this risk would be offset by the benefit provided by an examination with greater preoperatively localization accuracy and therefore allow for minimally invasive surgery.60
Among other drawbacks are the contraindications related to the use of iodinated contrast in allergic patients or those with renal involvement (remember that PHP can affect renal function), and the time required for the interpretation of the images.52,63 Also, its ability to detect adenomas may be limited by the small size of parathyroid adenomas, beam-hardening artefacts in the lower neck due to the clavicles and shoulders (which can be diminished if the patient lowers the shoulders or a rolled towel is placed between the scapulae) or motion artefacts from swallowing, talking, or coughing during image acquisition52 (Table 2).
CT is considered superior to ultrasound and scintigraphy as it provides accurate anatomical information as well as functional information,20,30,52,60 and is a quick study to perform61 (Table 2). Therefore, despite the fact that it was considered second-line for discordant or inconclusive cases,19 many authors believe that it could be an accurate first-line imaging modality.11,12,27,52
Magnetic resonanceThere are few publications about the usefulness of MRI in the preoperative location of PHP lesions. Generaly, MRI is used in patients with persistent or recurrent PHP61 Parathyroid adenomas usually present an intermediate signal similar to that of muscle on T1-weighted sequences, and are usually hyperintense on T2-weighted images, restricting diffusion. Dynamic studies using T1-weighted sequences with contrast (after injection of 0.1 mmol/Kg of gadolinium at a speed of 4 mL/s), show early contrast uptake a few seconds after the start of carotid enhancement and with maximum signal intensity in the early venous phase unlike thyroid nodules or lymph nodes, which enhance in the late phase.62–64
The sensitivity for the diagnosis of parathyroid adenoma is similar when using 1.5T (96.7%)62 or 3T (91%−92%).63,65 However, there are greater differences in terms of specificity, since 66.6%62 have been reported if 1.5T is used, versus 95% if 3T is used65 (Table 1). This could be due to the fact that Nael et al. carried out perfusion studies using TWIST sequences. They obtained that a Time to peak (TTP) threshold of 37 s allowed differentiating a single parathyroid adenoma from the thyroid gland with a sensitivity of 86%, as well as that a wash-in greater than 5.27 showed the highest specificity (90%) for differentiating a single parathyroid adenoma from the thyroid gland. A combination of TTP (threshold of 30 s), wash-in (threshold of 5.86) and washout (threshold of 0.67) improved the diagnostic power, resulting in an AUC of 0.96 with a sensitivity of 91% and specificity of 95%.65
Lastly, the use of MR spectroscopy has also been described. In this technique, increased levels of choline, glycerophosphocholine, phosphorylcholine, glucose, lactate, succinate, glutamine and ascorbate are associated with parathyroid adenomas but not with hyperplasia.66
This technique has shown similar sensitivity and specificity to multiphasic CT, with no risk of radiation.62–64 However, images may be artefacted by respiratory motion, esophageal motility, or cardiac pulsation.62 In addition, MRI is a test that requires a long acquisition time, is expensive, and has lower spatial and temporal resolution.62–64 Besides, neck anatomy could difficult an adecquate fat-suppression. The Dixon technique is recommendated in order to improve it.65
For all the above, MRI is considered a second-line imaging modality when the first-line imaging modalities are negative or inconclusive62–64 (Table 2).
PETParathyroid adenomas incidentally found using 18F-choline PET/CT while evaluating patients with prostate cancer have recently been described. There are some preliminary studies regarding the use of 18F-choline PET/CT in patients with PHP and without prostate cancer that affirm that this technique can locate parathyroid adenomas.67 Choline serves as a precursor for the biosynthesis of phospholipids therefore cells with a high rate of cell proliferation, such as those of a parathyroid adenoma, have a greater demand for phospholipids. Cells absorb choline and choline kinase enzymes phosphorylate it, leaving it retained inside the mitochondria in a similar way to sestamibi in oxyphilic cells. The high early uptake by parathyroid adenomas is due in part to high vascularization and increased phospholipid-dependent protein kinase activity.9,67,68 This has generated a lot of interest in recent years and there are many publications stating that this technique has a sensitivity of 81%–95.8%, a specificity of 12.5%–99.7% and an accuracy of 56.8%−95.3%21,24,25,51,69–72 (Table 1). It is known that, on static images, lymph nodes can also show uptake, leading to false positives. However, dynamic studies have found that discrimination between adenomas and lymph nodes is optimal at between 2 and 5 min after isotope injection.68
PET has the advantage of being much faster and having higher spatial resolution than SPECT.22,70 Among its drawbacks are its high cost, 3−4 times higher than a SPECT/CT with 99m Tc-sestamibi,34,72 and radiation exposure of around 9.5mSv because it is coupled with CT.9 For these reasons it is currently used as a second-line imaging technique.34 This radiation concern has led to the study of the use of 18F-choline PET/MRI, with similar success rates, while minimising the effective radiation dose to 2.5–3.8 mSv9,34 (Table 2).
C11-methylmethionine PET/CT has also been investigated with similar diagnostic yields with a sensitivity, specificity, and accuracy of 75%, 50%, and 71%, respectively. However, the short half-life of methionine limits its daily applicability5,17,69,71,73,74 (Table 1).
ConclusionTo summarise there is great variability between the different tests used for the preoperative localization of parathyroid pathology. The importance of knowing the different diagnostic options lies in the need to choose the most suitable test at each moment and for each patient in order for an adequate management of PHP patients with surgical criteria.
We must bear in mind that multidisciplinary management between surgeons, endocrinologists, nephrologists, radiologists and nuclear medicine physicians is important, especially in cases of discrepancy, since an adequate location determines a minimally invasive surgery with fewer risks, fewer number of complications and lower costs.
FoundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors would like to thank all the individuals who participated in this study for their willingness to collaborate.