Adenoid cystic carcinoma (ACC) of the breast is a rare tumour that represents less than 0.1% of breast carcinomas. It has a triple-negative phenotype; however, it is associated with a benign course, and both lymph node involvement and distant metastasis are rare. We present seven cases of ACC of the breast diagnosed in our centre. We describe their radiological characteristics, and specifically highlight the findings from magnetic resonance imaging.
El carcinoma adenoide quístico (CAQ) de la mama es un tumor poco frecuente que representa menos del 0,1% de las neoplasias mamarias. Presenta un fenotipo triple negativo; sin embargo, se asocia a un curso benigno siendo rara la afectación ganglionar y metastásica a distancia. Presentamos 7 casos de CAQ de la mama diagnosticados en nuestra institución clínica, con el objetivo de describir sus características radiológicas resaltando especialmente los hallazgos por resonancia magnética.
Adenoid cystic carcinoma (ACC) is defined as a tumour with proliferation of epithelial and myoepithelial cells. Its most common location is in the salivary glands, but it can also occur in other organs including the breast, lacrimal glands, ear canal, skin, larynx, tracheobronchial tree, prostate, cervix and Bartholin’s glands.1
ACC of the breast is rare, accounting for less than 0.1% of all breast cancers.2 It mostly affects postmenopausal women, and typically manifests as a palpable, mobile, well-circumscribed mass, sometimes with associated mastalgia,1–4 without a tendency to be bilateral.1,4
Immunohistochemistry shows a triple negative phenotype, due to its negativity for oestrogen receptor (OR), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), and low expression of Ki-67.5–8 It is associated with a more benign course than other triple-negative tumours and extra-mammary ACC, with distant lymph node and metastatic involvement being rare.5
Surgical resection is the standard of care. However, due to its low frequency, there are no clear guidelines on the selection of the surgical approach and the role of radiotherapy and adjuvant chemotherapy.7
We describe the radiological findings of a series of patients with ACC of the breast diagnosed at our hospital, in particular highlighting the magnetic resonance imaging (MRI) findings.
Case seriesWe retrospectively reviewed the results of ultrasound-guided core needle biopsies performed in our department from January 2006 to August 2024. Of the 5827 biopsies, seven cases were found with a diagnosis of ACC (0.12%). In five of them, MRI studies were available, performed with 1.5 T to 3 T equipment, using axial T2, dynamic axial T1 (one baseline and five obtained after injection of gadolinium in bolus of 0.1 mmol/kg, with subtraction of the post-contrast images), bilateral axillary coronal T1, diffusion and apparent diffusion coefficient (ADC) mapping.
We collected information on the radiological features from ultrasound, mammogram and MRI, and the histological findings after analysis of the surgical specimen (Figs. 1–3). This information is set out in Table 1.
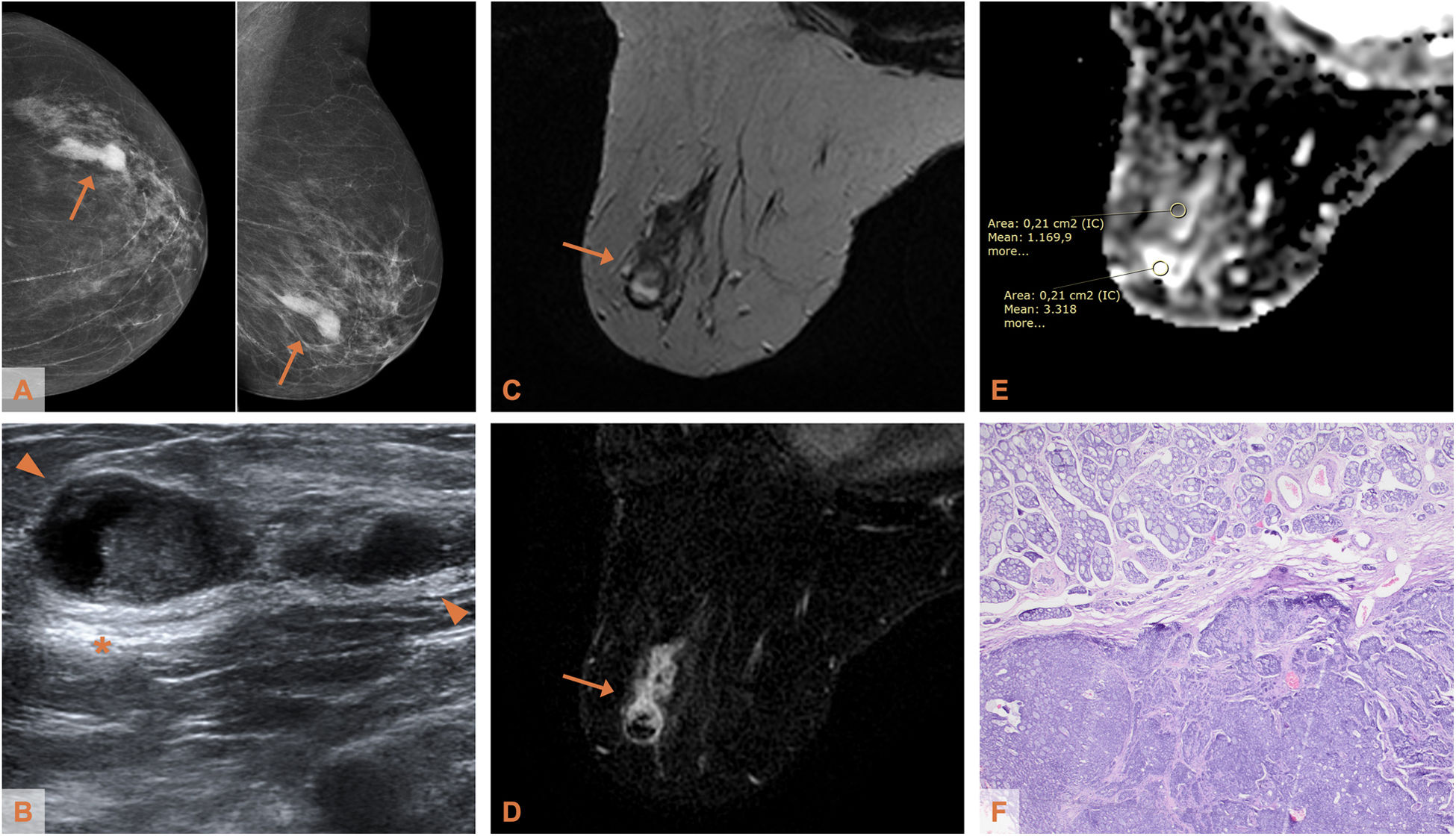
Adenoid cystic carcinoma of the breast in a 75-year-old woman (patient 1). A) Mammogram (craniocaudal and mediolateral oblique projections - CC and MLO): hyperdense lesion of irregular morphology in the lower outer quadrant of the left breast. B) Ultrasound: the lesion (delimited by arrowheads) shows complex echostructure (solid and cystic) and posterior acoustic reinforcement (*). C) Axial T2-weighted MRI: the lesion, with irregular morphology and non-circumscribed margins, is heterogeneous, predominantly isointense with respect to the parenchyma with hyperintense areas indicating the existence of a cystic component, depending on the histological type of the lesion. D) Axial T1-weighted MRI with post-contrast subtraction: heterogeneous mass-like enhancement, with ring-shaped uptake area. (E) ADC map: the apparent diffusion coefficient in the cystic component is 3.318 × 10−3 mm2/s and in the heterogeneous zone it is 1.169 × 10−3 mm2/s. F) Photomicrograph of the surgical specimen (haematoxylin-eosin stain, ×4): tumour proliferation with double component, cribriform (upper half of the image) and solid (lower half of the image).
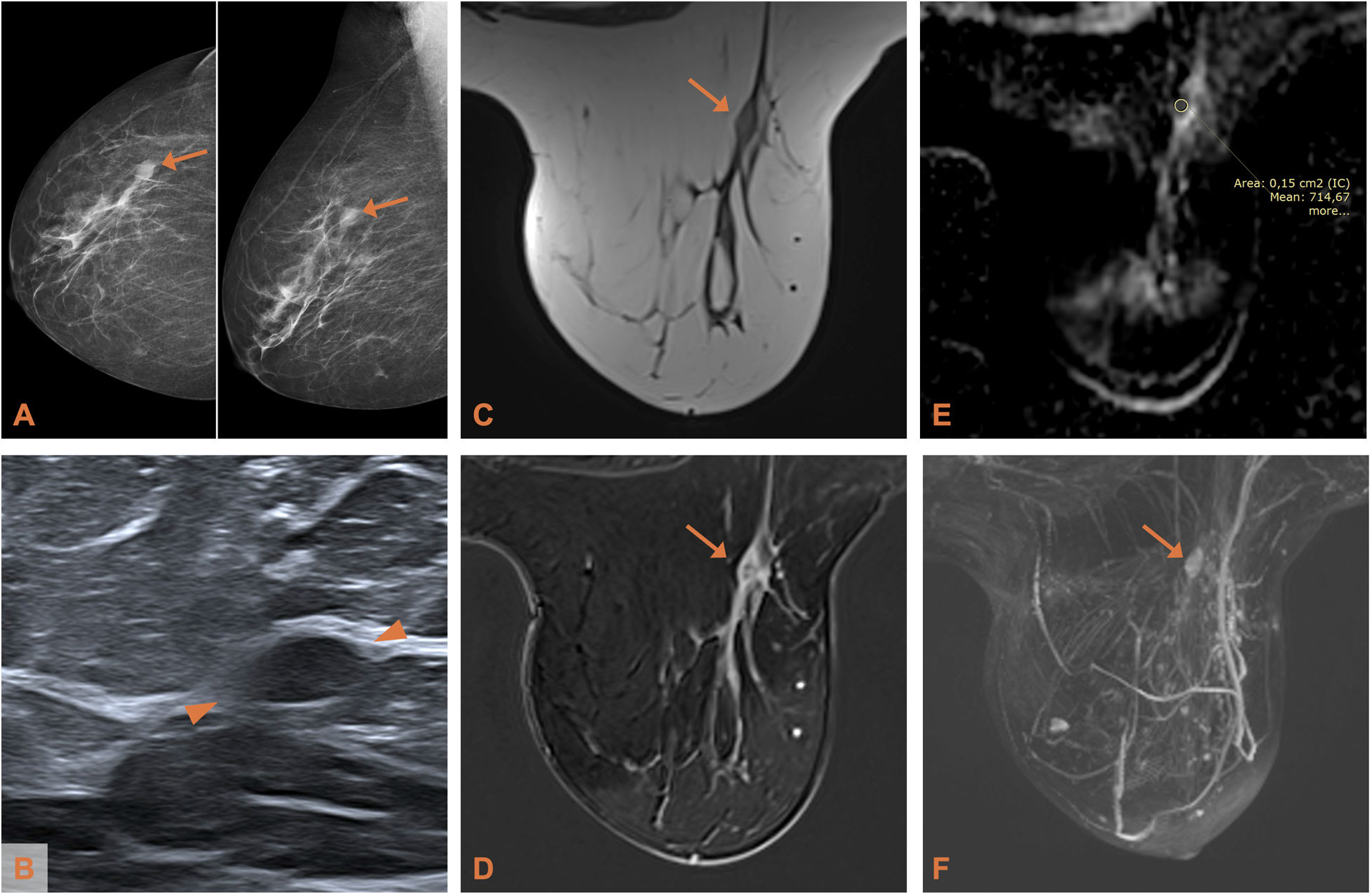
Adenoid cystic carcinoma of the breast in a 64-year-old woman (patient 2). A) Mammogram (CC and MLO projections): hyperdense lesion of irregular morphology at the junction of the outer quadrants of the right breast. B) Ultrasound: the lesion (delimited by arrowheads) has non-circumscribed margins and heterogeneous echostructure. C) Axial T2-weighted MRI: the lesion, with irregular morphology and margins, is slightly hyperintense with respect to the parenchyma, with hypointense fine septa. D) Axial T1-weighted MRI with post-contrast subtraction: heterogeneous mass-like enhancement. (E) ADC map: the apparent diffusion coefficient is 1.557 × 10−3 mm2/s. F) Photomicrograph of the surgical specimen (haematoxylin-eosin stain, ×4): predominantly cribriform tumour proliferation, with nests arranged over a dense fibrous stroma.
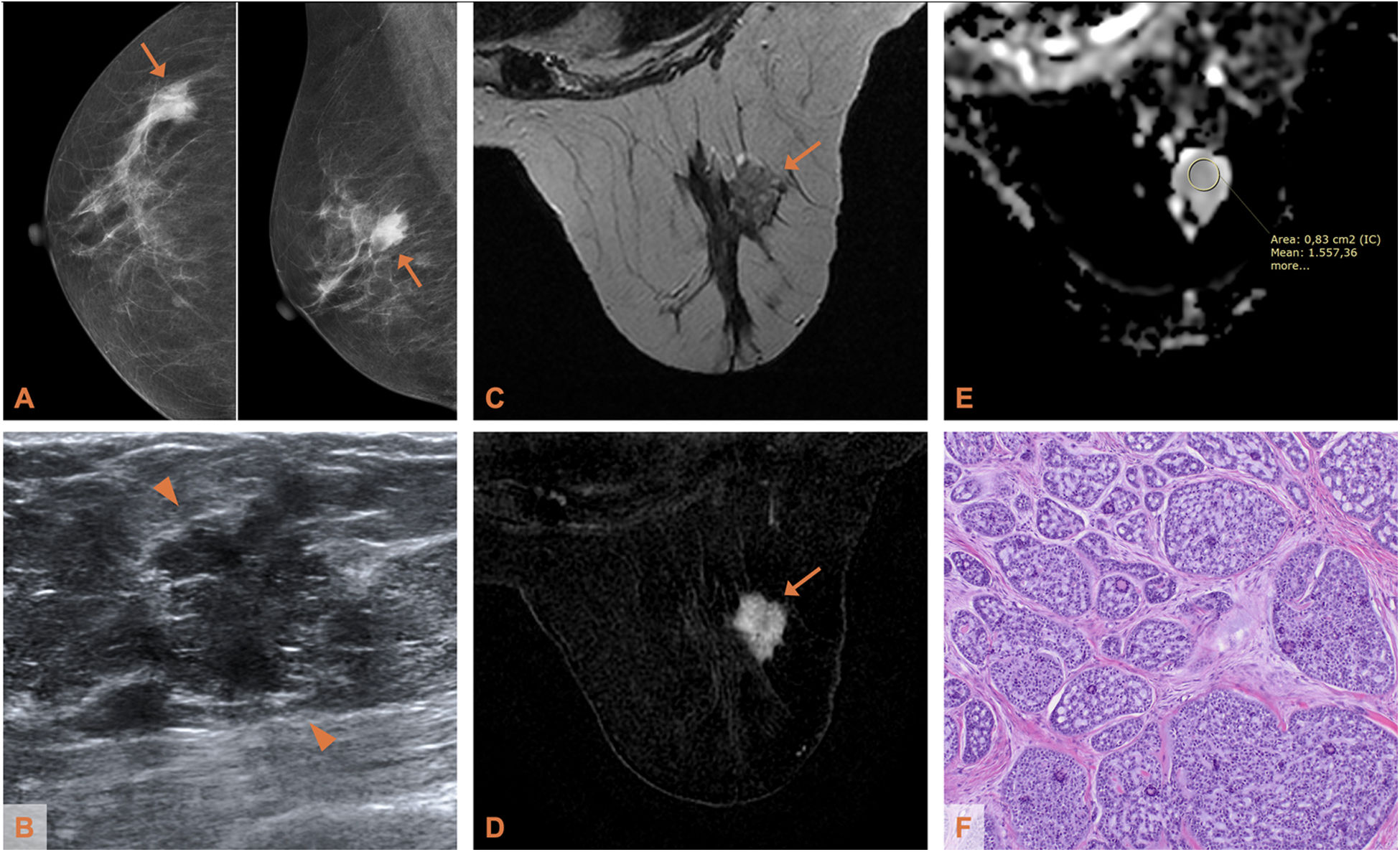
Adenoid cystic carcinoma of the breast in a 54-year-old woman (patient 4). A) Mammogram (CC and MLO projections): isodense lesion of oval morphology in the upper outer quadrant of the right breast. B) Ultrasound: the lesion (delimited by arrowheads) shows a slightly hypoechoic echostructure. C) Axial T2-weighted MRI: the lesion, with an oval morphology and circumscribed margins, is isointense with respect to the parenchyma. D) Axial T1-weighted MRI with post-contrast subtraction: heterogeneous mass-like enhancement. (E) ADC map: the apparent diffusion coefficient is 0.714 × 10−3mm2/s. F) Maximum intensity projection reconstruction (MIP) showing the lesion described.
Radiological and histological findings of the reviewed cases.
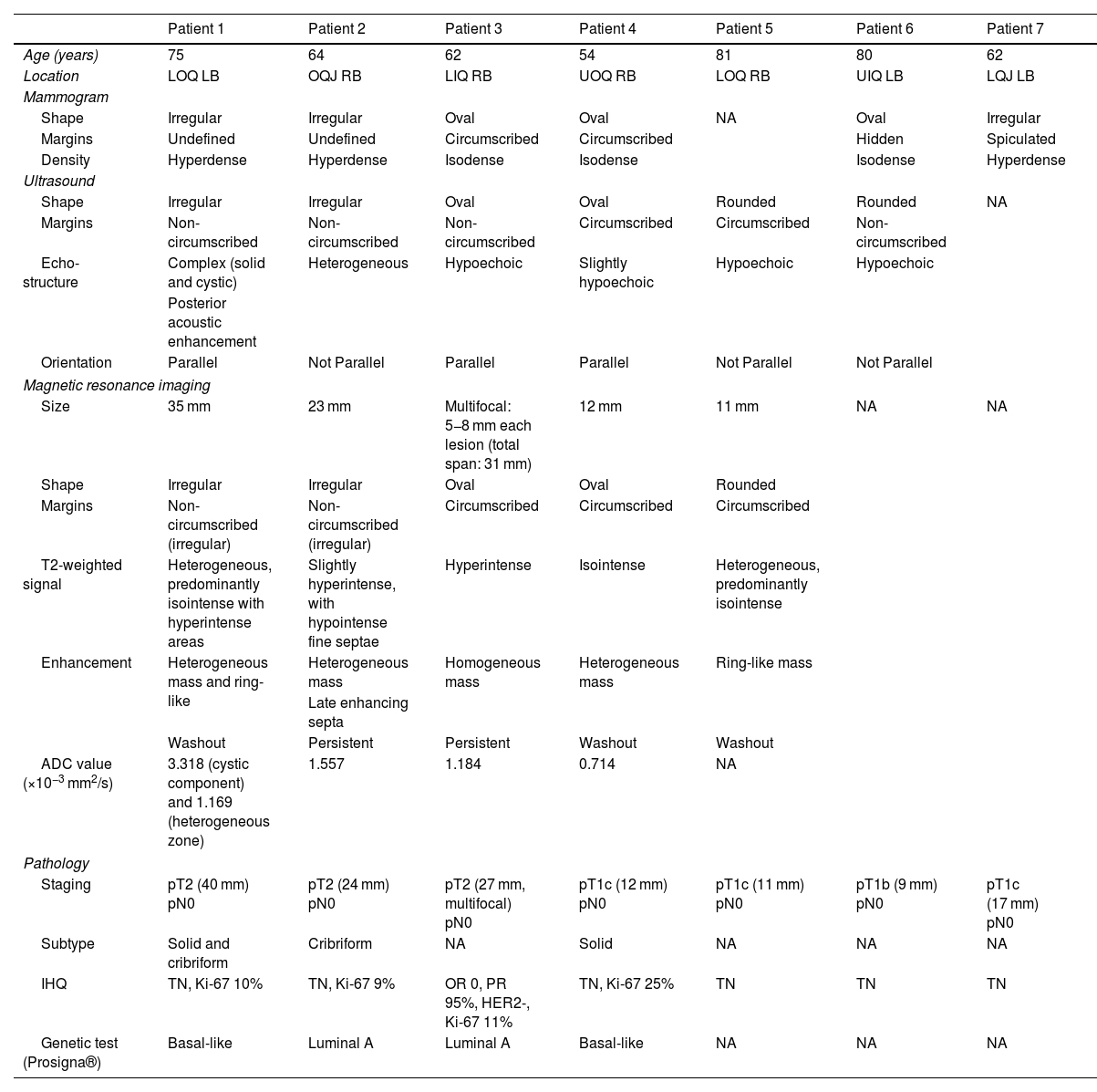
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Age (years) | 75 | 64 | 62 | 54 | 81 | 80 | 62 |
| Location | LOQ LB | OQJ RB | LIQ RB | UOQ RB | LOQ RB | UIQ LB | LQJ LB |
| Mammogram | |||||||
| Shape | Irregular | Irregular | Oval | Oval | NA | Oval | Irregular |
| Margins | Undefined | Undefined | Circumscribed | Circumscribed | Hidden | Spiculated | |
| Density | Hyperdense | Hyperdense | Isodense | Isodense | Isodense | Hyperdense | |
| Ultrasound | |||||||
| Shape | Irregular | Irregular | Oval | Oval | Rounded | Rounded | NA |
| Margins | Non-circumscribed | Non-circumscribed | Non-circumscribed | Circumscribed | Circumscribed | Non-circumscribed | |
| Echo-structure | Complex (solid and cystic) | Heterogeneous | Hypoechoic | Slightly hypoechoic | Hypoechoic | Hypoechoic | |
| Posterior acoustic enhancement | |||||||
| Orientation | Parallel | Not Parallel | Parallel | Parallel | Not Parallel | Not Parallel | |
| Magnetic resonance imaging | |||||||
| Size | 35 mm | 23 mm | Multifocal: 5−8 mm each lesion (total span: 31 mm) | 12 mm | 11 mm | NA | NA |
| Shape | Irregular | Irregular | Oval | Oval | Rounded | ||
| Margins | Non-circumscribed (irregular) | Non-circumscribed (irregular) | Circumscribed | Circumscribed | Circumscribed | ||
| T2-weighted signal | Heterogeneous, predominantly isointense with hyperintense areas | Slightly hyperintense, with hypointense fine septae | Hyperintense | Isointense | Heterogeneous, predominantly isointense | ||
| Enhancement | Heterogeneous mass and ring-like | Heterogeneous mass | Homogeneous mass | Heterogeneous mass | Ring-like mass | ||
| Late enhancing septa | |||||||
| Washout | Persistent | Persistent | Washout | Washout | |||
| ADC value (×10−3 mm2/s) | 3.318 (cystic component) and 1.169 (heterogeneous zone) | 1.557 | 1.184 | 0.714 | NA | ||
| Pathology | |||||||
| Staging | pT2 (40 mm) pN0 | pT2 (24 mm) pN0 | pT2 (27 mm, multifocal) pN0 | pT1c (12 mm) pN0 | pT1c (11 mm) pN0 | pT1b (9 mm) pN0 | pT1c (17 mm) pN0 |
| Subtype | Solid and cribriform | Cribriform | NA | Solid | NA | NA | NA |
| IHQ | TN, Ki-67 10% | TN, Ki-67 9% | OR 0, PR 95%, HER2-, Ki-67 11% | TN, Ki-67 25% | TN | TN | TN |
| Genetic test (Prosigna®) | Basal-like | Luminal A | Luminal A | Basal-like | NA | NA | NA |
ADC: apparent diffusion coefficient; HER2: human epidermal growth factor receptor 2; IHQ: immunohistochemistry; LB: left breast; LIQ: lower inner quadrant; LOQ: lower outer quadrant; LQJ: lower quadrant junction; NA: not available; OQJ: outer quadrant junction; OR: oestrogen receptors; PR: progesterone receptors; RB: right breast; TN: triple negative; UIQ: upper inner quadrant; UOQ: upper outer quadrant.
ACC consists of a proliferation of two cell types: myoepithelial cells (basaloid cells) clustered in solid tumour nests or lining cribriform spaces (pseudocysts); and epithelial cells lining true glandular lumens containing Schiff's periodic acid-positive material.4,6,7 There are three architectural patterns, and it can present in a mixed form: cribriform, solid and tubular-trabecular, the first being the most common, and the second the most aggressive.1,5,7,8 In terms of phenotype, although they have traditionally been classified as triple negative according to the immunohistochemistry results, in our series the genetic tests reveal that some of them belonged to the luminal subtype.
Radiological findings of ACC are usually non-specific. On mammogram it is defined as a lobulated or irregular lesion, with variable margins (circumscribed, microlobulated, indefinite or spiculated), as an architectural distortion or as a developing asymmetry. Calcifications are rare.2–4,6 On ultrasound, it appears as a lesion of variable shape (round, oval, irregular), with generally non-circumscribed margins, arranged parallel to the skin. The ultrastructure is typically hypoechoic or heterogeneous, or solid-cystic complex in larger lesions1,3,6 although cases of isoechoic lesions have been reported.9 They may show posterior acoustic enhancement, but rarely show echogenic halo or posterior acoustic shadow.1,3,6,9 The cases in our series are broadly consistent with those reported in the literature.
As for MRI findings, to date only a few individual case reports and three series with small sample sizes of up to nine cases have been published.3,6,9 The most typical feature of breast ACC, according to these studies, is the intermediate to high signal on T2-weighted images (hyperintensity or isointensity with respect to breast tissue). In our study two were hyperintense, one was isointense and two were heterogeneous, predominantly isointense with hyperintense foci. Signal hyperintensity is most often found in benign breast lesions, such as cysts, complicated cysts or myxoid fibroadenomas, but can also be seen in mucinous carcinoma, due to the high mucin content, and in high-grade infiltrating ductal carcinoma with central necrosis. In contrast, malignant lesions generally have a lower signal intensity on T2-weighted images.10
Tang et al.3 have described the presence of internal septa showing low signal on T2 and late enhancement in ACC, especially in larger lesions. This is consistent with one of the cases in our series, which had thin septa on hypointense T2-weighted images with late-phase uptake. This study suggests that these internal septations may correspond to the stroma embedded between the tumour islets.
In terms of the shape and margins on MRI, the presentation is variable. They are described in the literature as oval, rounded or irregular nodules, with circumscribed or non-circumscribed margins. Some series3,9 lean towards benign characteristics. In our study we can see that the variability in morphology may be related to size. The three smaller lesions (∼10 mm) have features suggestive of being benign. In contrast, the two larger lesions (>20 mm) have irregular morphology and borders.
Four of the patients in our series had a diffusion study. Diffusion can be quantified with the ADC value. This tends to be low in high cellularity lesions and helps to distinguish between benign and malignant lesions. In two of the four lesions analysed, restricted diffusion was observed, in particular in the smaller lesions, probably due to the predominance of the solid component. In contrast, the larger lesion, which had a double component in the morphological sequences, showed restricted diffusion in the heterogeneous component and high diffusion in the cystic component. In the literature, we only found a single series of ACC9 which also has a diffusion study for some of its cases, and which shows mostly (71%) restricted diffusion.
Looking at contrast uptake by the lesions, they show a mass-like enhancement with a variable pattern, between heterogeneous, homogeneous and ring-like, and the time-intensity kinetic curves are also variable, from persistent enhancement to washout.2,3,6,9
ConclusionsACC of the breast is a very rare tumour with variable radiological features according to the findings of our series and those published in the literature. However, we found that the morphology of these lesions could be related to their size, showing an intermediate to high signal on T2-weighted MR images and tending to be restricted in the diffusion study.
CRediT authorship contribution statement- 1
Responsible for the integrity of the study: MF, IV, DG, AM and XB
- 2
Study conception: MF and XB
- 3
Study design: MF and XB
- 4
Data collection: MF, IV and XB
- 5
Data analysis and interpretation: MF, IV and XB
- 6
Statistical processing: not applicable
- 7
Literature search: MF
- 8
Drafting of the article: MF and XB
- 9
Critical review of the manuscript with intellectually relevant contributions: MF, IV, DG, AM and XB
- 10
Approval of the final version: MF and XB
This study received no specific grants from public agencies, the commercial sector or non-profit organisations.