Pulmonary radiofrequency ablation requires more than just interventional radiology skills. Patients must be selected carefully, and the acts that need to be done before, during, and after the procedure must be coordinated. To guarantee patient safety, radiologists need to know the variants of the technique, the precautions that must be taken, the complications that can occur, and the risks involved. Early differentiation between tumor tissue and normal changes secondary to treatment on imaging tests will make it possible to repeat the treatment without delays, and this will increase survival. This article describes how to coordinate and carry out pulmonary radiofrequency ablation, the complications of the technique, and the current evidence in follow-up.
Para la ablación pulmonar con radiofrecuencia no solo son necesarias habilidades intervencionistas. Tras seleccionar adecuadamente al paciente, hay que coordinar las actuaciones previas, durante y posteriores al procedimiento. Conocer las variantes de la técnica, las precauciones, las complicaciones, los riesgos y las recomendaciones para el seguimiento garantizará la seguridad del paciente. Diferenciar precozmente en las pruebas de imagen el tejido tumoral de los cambios normales secundarios al tratamiento permitirá volver a tratar pronto al paciente, lo que aumentará su supervivencia. El objetivo de este trabajo es describir cómo coordinar y realizar la ablación pulmonar con radiofrecuencia, sus complicaciones y la evidencia actual en el seguimiento.
Radiofrequency ablation (RFA) is a current alternative to treat localized lung neoplasms with concrete recommendations.1,2 It is important to know the procedure and coordinate the process actions from selecting the patient adequately in a multidisciplinary committee that ensures the best therapeutic alternative possible to performing the RFA without delay but with minimal risks and complication impact. It is essential to make a follow-up of the lesion through a method that can detect local tumor persistence early that treated with RFA improves survival.3 Tumors treated through ablation remain “in situ” and they will undergo dynamic changes secondary to therapy for a long time. Distinguishing them from tumor persistence or recurrence is a complex challenge and it can be done with image modalities only.4–6 The type of test, the timing, the technical protocol and the right interpretation will be crucial. Our goal is to describe how to coordinate and perform the pulmonary radiofrequency procedure, get to know its complications and the current evidence during follow-up.
Actions prior to the proceeding- 1.
Determining the optimal therapy in multidisciplinary session7 with the oncologist, a radiologist experienced in pulmonary procedures, a radiotherapist, a chest surgeon and a pneumologist.
- 2.
Assessing the cardiopulmonary situation and the risk of bleeding and correcting possible contraindications.2 Warfarin and acenocoumarol should be withdrawn 4–5 days earlier8 though there is no solid evidence to withdraw antiplatelets,9 it is recommended that acetylsalicylic acid, clopidogrel and non-steroidal anti-inflammatory drugs to be withdrawn 5–7 days earlier,1,7,10,11 as well as the rest of antiaggregants based on their pharmacodynamic properties. In procedures that are not complex, one prior therapeutic dose of low molecular weight heparin of subcutaneous absorption or derivatives should be withdrawn an in complex procedures 2 doses.11 Pulmonary spirometry is useful in patients with diffuse lung disease or lung surgery but it is not performed systematically.12 The patient should be debriefed in a pre-anesthetic meeting.
- 3.
Excluding the disseminated disease and assessing the need to confirm malignancy. A CT is recommended in the month prior to the RFA to stage the neoplasm, establish, according to its size, location and risks, whether RFA can be used and the approach plan.13 Performing a PET-CT or MRI before the procedure will depend on the primary tumor.12 PET-CT is especially useful to detect adenopathies and hematogenous metastases with a knack for fluorodeoxyglucose such as those of breast and colon,14 which would change the therapeutic strategy.15 It is recommended to study the lesion anatomocytologically if there is suspicion of lung primary tumor,16,17 but with enough time to solve an eventual post-puncture hemorrhage that would interfere with the performance of RFA15,18 and evaluate its immediate result. We recommend performing it the same day as the CT prior to the RFA. It is not generally necessary to confirm a metastasis anatomocytologically if its radiological appearance is typical.17 It is useful to obtain informed consent in the weeks prior to the procedure to make it clear that serious complications are exceptional19,20 and insist on the fact that patients should take their medication the very morning of the procedure-anti-hypertensives, heart medication and half their usual dose of insulin.19 It is usually recommended not to treat lesions on both lungs at the same time and to space their therapy weeks apart to avoid serious simultaneous bilateral complications such as hemorrhages or pneumothorax. Studies from experienced centers but with a moderate sample size (n=27) consider it feasible in selected cases.21 It is possible though to treat several unilateral lesions in the same session.12
Although the effectiveness of prophylactic anti-biotherapy has not been confirmed the devitalized tissue can be a focus of infection.16 Some authors recommend it systematically22 and others preoperatively in patients with valvular or articular prostheses and postoperatively in patients with reduced mobility or COPD, to reduce the risk of pneumonia.1,10 It should be performed 30–60minutes through the patient's IV. The patient should take off all jewellery and metallic objects.10 The patient is placed on the CT table following instructions from the radiologist as if a lung biopsy was to be performed. The CT is the only accurate modality as a pulmonary image guide.12,20,23 The supine or prone position reduces the risk of movement and displacement of the electrode with respect to the lateral or oblique positions.10 The lower the table the more space within the gantry.10 To reduce the risk of burns the conductive pads are placed on the legs, with the legs separated, or on the thoracic wall contralateral to the area to be treated contacting the skin in all its surface, without any folds and as equidistantly as possible to the area to be treated. They should be checked every time the patient is mobilized and during ablation to prevent excessive heating.16 The vitals are monitored (oxygen saturation, respiratory rate, pulse, ECG, blood pressure) and oxygen is supplied through a mask.10,24 The CT is performed without IV contrast (IVC) to plan the needle exact trajectory so that the active tip stays at the center of the tumor following its longitudinal axis.19 The gantry can be angled to achieve a more certain intercostal access and avoid going through vessels, bronchi and sulci.10,16 Once the trajectory has been decided, 10ml of 1% local lidocaine is administered up to the extrapleural fat with CT control and the anesthetic technique is initiated. Conscious sedation is the most commonly used technique.10,25,26 Coordinating the respiratory pauses with the introduction of the needle14,17 and avoiding broad respiratory chest movements10 under general anesthesia facilitates the procedure, improves its tolerance12 and increases the percentage of ablation according to some authors.23 But the hospital stay1 and morbidity are greater14 due to its greater risk of exacerbating a pneumothorax,27 pulmonary hemorrhage and bronchopleural fistula (BPF).10 Some authors recommend it for difficult-to-access multiple tumors,12,17 in patients with compromised airways, high risk of bleeding or significant limitation of cardiopulmonary reserve-oxygen-dependence, aortic, carotidal stenosis, serious arterial coronary disease1,10 who would not tolerate ablation with conscious sedation.19 Double lumen endotracheal tubes should be used to protect the contralateral lung from a hemorrhage.1,17 For intravenous conscious sedation midazolam (0.07–0.08mg/kg), propofol (0.5–2.0mg/kg/h) and fentanyl (0.5–2.0mg/kg/h) are combined.24 The dose should be increased when the electrode is placed and during the first few minutes of ablation.10 The patient should remain still without talking or coughing to prevent complications and the electrode from moving.10 The pleural surface should be pierced once only.10 The active tip of the needle should pierce the nodule 0.5cm on each end.10 CT multiplanar reconstructions will be very useful to place the needle.12,16 Some authors recommend using CT fluoroscopy with real time images but the significant increase of radiation for both the patient and the professional should be taken into account.12 We will be able to treat <2cm lesions with a single ablation and lesions from 2 to 3cm with modalities that increase the ablation volume.2 For >3cm lesions it is adequate to assess other therapies.2 Piercing the tumor with the central axis of the expandable electrodes should be avoided to prevent tumor implantation.12 Its multiple active tips should surround the tumor and cover the safety margin. The rest of the needles on the neighboring skin should be withdrawn before the current passes. Today there are no official RFA protocols. The adequate impedance level,10,19 the number and duration of the cycles vary based on the specifications of each generator.16 The maximum temperature should range between 60 and 100°C.10 With the Cool-tip system the needle cooling system is activated and then the current passage under impedance control. It is recommended to start with low power in the lung (35W) and increase it gradually unlike we do with solid organs15,16 in order to avoid carbonization–it is translated into a dramatic increase of impedance. In case of carbonization the electrode should be repositioned moving it away from the carbonized tissue so that both the heat and the current can be diffused. The appearance of repeated impedance “peaks”-approximately 90 in 60s under power modulation indicates that the tissue surrounding the electrode has been treated successfully.20 At the end the effects of CT therapy are assessed.10,20 If the tumor is not surrounded completely by a 0.5–1cm “ground-glass” halo (GGH) or a temperature of 60°C has not been reached then another cycle should follow. The needle should be repositioned, keeping it “hot”, toward the area unsurrounded by a GGH10 without surpassing the pleura to reduce the risk of pneumothorax.10 When finishing the therapy we withdraw the electrode cauterizing the trajectory–unnecessary in expandable electrodes and assess complications through CT. The patient is placed with the treated hemithorax down to minimize the risk of pneumothorax10 and hemorrhage aspiration. Oxygenating the patient through a mask promotes the re-absorption of small pneumothorax.10 The patient should be monitored and pain controlled during reanimation for 2–3h.20,27 A supine chest X-ray is then taken postoperatively 1–4h later17,27 and a chest CT with and without IV C the following day to assess complications and the therapy outcome. If there are no complications the patient is discharged 24h after the RFA. There are discharge recommendations after 3–4h10,20 and X-ray control at 24h.19 A week later a posteroanterior and lateral chest X-ray is performed to check out the patient.4
Follow-upA. Why with image modalities?
In one RFA the tumor remains “in situ” and it is not possible to ensure complete tumor necrosis anatomopathologically or the absence of lymphatic dissemination. We can only assess the outcomes radiologically.4–6 The cytological study (puncture-aspiration) of the ablation margins can yield false negative results if it is conducted in an area without tumor cells, and instead yield false positive results due to the existence of dead tumor cells–in a hyperthermia-induced apoptosis process4 but with the erroneous appearance of feasibility since the tissue architecture is preserved after the ablation (phantom cells).4 The biopsy has more diagnostic values but still yields false negatives.4
Other to assessing the therapy immediate and evolutionary result image modalities are also supposed to detect complications,10,17,19 tumor dissemination and metachronic tumors.4
B. With what modality?
There are no guidelines about the ideal modality. CT and PET-CT are more frequently used.4,15,17–20,28 Many authors consider PET-CT to be more sensitive and specific for the assessment of response4,23,26,27 and they recommend it for the follow-up,4,5 but more research is necessary before we define its role.28 CT is more accessible.5 It should be performed with and without IV contrast before and after RFA18 for the evolutionary measurement and comparison of contrast uptake. Measuring the contrast dynamic uptake for a lesion through a densitometry CT (a reflection of its vascularization) theoretically speaking can be more sensitive to be able to differentiate benign from malignant lesions3–5,23,27 and detect early tumor tissue. It consists of a CT without IV C and sequential acquisitions with IV C (0, 45 [90], 180 and 300s).3–5 Images with maximum contrast uptake are obtained by subtracting the density of the lesion in the simple study from the maximum attenuation images of the lesion with contrast.4 It is not used much because it is complicated, useless in multiple or cavitated lesions3 and because it requires more radiation doses than conventional CT.
The MRI is not used much either because it is expensive, not widely available and limited when it comes to assessing the lungs.4 Experimental data indicate that diffusion is an early indicator of response.3–5,29
C. Interpretation of image modalities (Figs. 1–3).
Expected findings in CT after pulmonary radiofrequency ablation. Color code: light blue-translucent: ground-glass; dark blue: consolidation; dark gold: IV contrast uptake; light gold: no IV contrast uptake; gray-black: cavitation. The color of this figure can only be appreciated in the article electronic version. B: benign; F: frequency; m: month; RF: radiofrequency; w: week; Tm: tumor; GG: ground-glass.
CT findings suggesting therapy failure after pulmonary radiofrequency ablation. Color code: light blue-translucent: ground-glass; dark blue: consolidation; dark gold: IV contrast uptake; light gold: no IV contrast uptake. The color of this figure can only be appreciated in the article electronic version. Enhancement>10–15HU (>10HU in conventional CT and 15HU in densitometry CT). m: months; RF: radiofrequency; HU: Hounsfield units; GG: ground-glass.
Expected findings (
Tumor and peritumor tissues will undergo necrotizing dynamic processes, cytolysis and inflammatory changes days after the RFA that make the lesion grow.10 This makes it a challenge to be able to make a radiological differentiation between the normal evolution of an incomplete treatment and a tumor relapse5,6,19–more common than after a lobectomy.5 It is necessary to distinguish them early to go back to treat them again without any further delay. The classical sign of tumor growth is not useful. Enhancement in CT and the metabolic activity of PET-CT play an important role,3,5,19,23 though they are not tumor-specific either (Figs. 1 and 2).
1. Expected findings in TC (Fig. 1):
- -
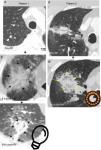
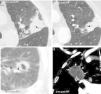
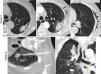
Ground-glass halo (GGH) and growth. Early indicators of therapeutic success14 (Figs. 4–7 and e-fig. 4). Immediately after the RFA the peritumor GGH appears. It is representative of coagulative necrosis but also of congestion, inflammation and hemorrhage.1 Three anatomopathological layers have been defined experimentally in GGH. The central layer has cells that have died of coagulative necrosis–cytoplasm with condensed chromatin nuclei and the intermediate one has alveolar lumens filled with liquid.4,20 In the peripheral one, of 2.6–4.1mm, made up of areas of non-necrotic hemorrhagic congestion and neutrophil infiltration viable tumors persist.4,6,20 This is why the GGH can overestimate the area of cellular death.4 For tumor necrosis to be considered complete the GGH should have a thickness of 5–10mm1 measured from the tumor margins.4,18,30 Because viable tumor cells can persist in the GGH peripheral layer6 the ideal is that it has a thickness of 15mm6 or an area four times larger than that of the tumor before performing the RFA.6,18,29 Signs of rosette, light bulb4 (Figs. 1, 4–6 and e-fig. 4) and inverted halo31 have been reported.
Figure 4.Early findings to be expected after success of an RFA (Radiofrequency Ablation) of two small lung lesions (a) and (d): important growth after RFA of the lesion in patient 1 (arrowhead in a) due to formation of a great ground-glass halo around the lesion (arrows in b). “Light-bulb sign” (light-bulb shaped opacity formed by the lesion treated and by the trajectory of the needle) at 24hours (h) (arrows in c) and “rosette sign” (concentric rings of different attenuation with inner ground-glass and dense outer ring), (arrows in e) at 14 days (d) after RFA in the second patient. This figure can be seen in more detail online (e-fig. 4).
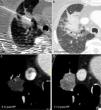
Figure 5.Example of successful therapy of a previously radiated nodule in the RUL (right upper lobe) with high SUV in the PET (not shown) indicative of relapse. (a) The size of the node (measurement taken in a) is eligible for radiofrequency (RF) therapy (<3cm). (c) Ground-glass area around the lesion after ablation of a right size (14mm). Absence of enhancement after ablation: the Hounsfield units (HU) do not go up in the analysis with IV contrast (IVC) (17HU) (e) with respect to the simple one (19HU) (d) one month after therapy (m). The lesion up-took contrast before the ablation (46HU) (b). (f) Good evolution after one month with transformation of the ground-glass area into consolidation that is larger than the lesion prior to the ablation (measurement taken in f). Relapse in lingula 7 months later (not shown).
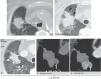
Figure 6.Example of successful therapy: small nodule in the left lower lobe (arrowhead in a) with concentric ring formation of different attenuation (“rosette sign”) 24hours (h) after performing the RF (arrows in b) and ground-glass (asterisks in b and c). The lesion grows the following day across the GGH (asterisks in c) which is of the right size to consider that the therapy has been successful (12mm). Residual scar lesion 17 months (m) later without any signs of relapse (d).
Figure 7.Findings expected after successful radiofrequency (RF) ablation. The lesion (arrowhead) grows extensively 24hours (h) after the RFA due to the appearance of a thick ground-glass halo around the lesion (upper images). Involution of ground-glass to a solid opacity 26 days later (d) without any focal uptakes of IV contrast (central images). Small residual scar lesion 17 months after the RF (lower image).
- -
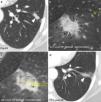
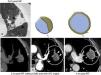
Cavitation. It is caused by devascularization of both the tumor and lung that in time communicate with the airway that is also necrotized. Peritumoral granulation tissue persists forming a capsule (Fig. 8 and e-fig. 4). It is sterile and does not require antibiotherapy.10 It is considered as a sign of positive response to the therapy.6
Figure 8.Expected findings after radiofrequency ablation (RFA) in different patients. Intralesion bubbles 24hours (h) after the RFA not present before the ablation (not shown) persistent after six months (m) (arrows in a and b). Cavitation after ablation that communicates with a bronchium (arrows in c) 22 days (d) after the RFA. Fine, soft-edged ring enhancement around the ablation (benign), (arrows in d).
- -
Gas bubbles in the lesion (Fig. 8).
- -
Benign enhancement around the ablation. Concentric with soft edges and <5mm, unlike an abscess whose wall would be thick and hyperuptaking (54). There should not be central or nodular uptake (Fig. 8).
- -
Fewer uptake than before the RFA (Fig. 5).
- -
Pleural effusion or thickening, hilar or mediastinal adenopathies.
The PET findings are described in Fig. 3.
2. Findings suggesting therapy failure (Figs. 2 and 3).
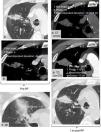
Treatment would be incomplete if the following signs appear: If GGH is incomplete or <5mm thick (Figs. 9–11)4,5 or if the lesion does not grow right after RFA or if it enhances in a nodular or irregular manner centrally or eccentrically.
Signs of unsuccessful therapy and relapse; 24hours (h) after the radiofrequency ablation procedure (RFA) (a) a narrow, incomplete ground-glass halo is formed surrounding the posterior and lateral areas of the lesion (black asterisks in (b), but not in the anterior pole (white asterisk in b). Eccentric nodular uptake in the anterior pole barely visible after two months (m) (arrow in c) but evident after 11 months (arrow in d)–moment in which the lesion grows eccentrically too.
Example of poor evolution after radiofrequency ablation (RFA). >3cm tumor (measured in a), with incomplete ground-glass halo (black arrowheads in b) 24hours (h) after the RFA. Eccentric growth of the lesion (white arrowhead in c) uptaking contrast in a nodular manner after 4 months (regions of interest–ROIs), with an increase of 70 Hounsfield units (HU) between the simple analysis (d) and with IV contrast (IVC) (e) indicative of relapse.
Example of poor evolution after radiofrequency ablation (RFA). >3cm tumor in contact with the sulcus (arrowheads in a). After the RFA the ground-glass halo is not formed around the lesion (a). Compared with the study without IV contrast (IVC) (b) after the administration of IV C it is possible to observe eccentric IVC uptake after 2 months (m) (white arrowheads in c) and in thick rings after 4 months (m) (golden arrowheads in d) moment in which the lesion grows too.
Eccentric growth10 and global growth from the third month onwards23 and usually eccentric enhancement, >10HU in standard CT with IV C3 and >15HU in the densitometry CT without the characteristics of benign enhancement around the ablation suggest tumor (Figs. 9–11). The PET-CT shows a reduction of metabolic activity <60% compared to before therapy4 usually eccentric or a SUV (Standardized Uptake Value) >3 from the 2–3 month onwards.3,4,18,23 A 1.5 SUV at 3–9 months shows a 77.8% relapse sensitivity and a 85.7–90.5% specificity. This is why some authors recommend bringing the threshold a little lower.10,15,18 These lesions should be followed up more closely through CT with and without IV C or biopsies should be performed.10,18
3. Response and progression/relapse criteria.
The classic RECIST criteria on which tumor progression is based in growth do not work when it comes to assessing the RFA immediate outcome.14,23,27 Herrera et al.33 proposed modified RECIST criteria for CT and PET-CT (Fig. 12) that assess the size, appearance and metabolic activity of the lesion.14,20,27,34 The WHO criteria are less indexed and also include enhancement and distance dissemination27 (Fig. 12).
4. MRI findings.
Coagulative necrosis is hyperintense in T1 (or isointense to the lesion before the RFA)10 and hypointense in T2. Peripherally the lesion can be hyperintense in T2 due to congestion and inflammatory changes in the neighboring lung.10 The absence of uptake that existed previously suggests complete ablation. The ablation area should surpass the tumor margin in 1cm. A thin uptake ring can be seen as a normal finding. The focal nodular tissue in the ablation margin with signal and uptake characteristics similar to the original tumor suggest residual tumor.25 Diffusion is promising for the early detection of the disease. A study showed that the apparent diffusion coefficient 3 days after performing the RFA was significantly greater in patients with progression at 6 months.32
D. When?
It will be essential to perform a CT approximately a month after performing the RFA, as a basal study and then in 3-month intervals as recommended by the Technological Committee of the Society of Intervenional Radiology.18 The best time to perform the PET is not clear.28 The inflammatory changes occurring the following weeks can increase the IV C uptake and the metabolic activity in PET-CT23 (Fig. 1) which is a source of false positives. Although some authors defend performing a PET-CT in the first few hours (<48h or ≤4 days)19,28 to detect residual tumor before the inflammatory changes appear most of them recommend not performing it before one month12 or even three months4 since the metabolic hyperactivity of inflammatory causes does not stabilize until two months have gone by (Fig. 3). Tumor progression is usually detected through CT and PET-CT between 2 and 6 months after performing the RFA, when the inflammatory changes have decreased.5,10
All authors recommend performing follow-up CT, densitometry CT or PET-CT 1–2, 3, 6, 9 and 12 months later. During the second year recommendations are variable.3–6,10,14,15,20,26,27,30,35 While some recommend performing CT and/or PET-CT every 3 or 6 months others suggest alternating them every 3 months. Two recent papers4,6 recommend performing CT6/densitometry CT4 every 6 months and others recommend performing densitometry CT every 3 months and PET-CT every 6.5 Afterwards it is recommended to keep an annual follow for 5 years.1 Relapses can occur once the PET18 metabolic activity is gone and this is why the follow-up should be long.4 Our recommendations can be seen in Fig. 12.
The few existing publications on MRIs recommend performing it 1–2 weeks before the RFA and a week later.25
Coordinating the specific actions to be implemented around the procedure has been schematized as a clinical pathway in our center (e-table 1).
ComplicationsIts frequency and risk factors are presented in e-table 2. They are common (50–60%), varied, but most are mild and self-limited. On occasion they evolve into greater complications.
A. Pleural.
- -
Pneumothorax (Fig. 12). It is the most common complication in all ablative modalities. It is usually asymptomatic. It generally develops in the two hours following the RFA.20 It needs to be drained when increases in successive X-rays, if there are symptoms or emphysema.10 Late pneumothorax due to subpleural necrosis or BPF is usually irrelevant6 but 12% required late management in one series.15 The time before diagnosis was 24±66hours. The ablation of the trajectory reduces the risk.27 It can last for 1–2 weeks as contained BPF.4
- -
Pleural effusion. The second most common complication in terms of frequency; usually small, self-limited and asymptomatic. It can be a response to thermal damage.36 It appears 1–2 weeks after the RFA.31 Though small it can be asymptomatic in patients with a single lung or serious chronic respiratory failure and require thoracocentesis or thoracostomy.
- -
Rare complications (≤1%):
Hemothorax. Potentially lethal if misdiagnosed. In the event of a rapidly growing pleural effusion during the procedure, with or without signs of hypovolemia arterial bleeding should be ruled out. It can be embolized.14
Bronchopleural fistula. It is presented as an untreatable pneumothorax. It develops during the first 4 weeks, while there is necrosis after the RFA.28 Most resolve spontaneously. They show an increased activity in the PET.10
Empyema.
B. Pulmonary.
- -
Cavitation. It also indicates a favorable response to therapy. Most are asymptomatic. Rare cases of hemorrhage and pneumothorax have been described due to rupture of the cavity.36
- -
Intra-parenchymatose hemorrhage. Caused by small vessel damage in the trajectory of the needle. It is associated with hemoptysis in a minority of cases (2–16.1%) usually small, self-limited and 2–7 days after the procedure.12 The risk of death is extremely rare – only 2 cases being reported,36 at least 1 related to the associated brachitherapy.3,7 To avoid it, it is recommended to keep the needle “hot” when repositioned or removed.37
- -
Pneumonia. Prophylactic use of antibiotics has not shown any improvements.12
- -
Pneumonitis (e-table 2).
C. Thoracic wall and vessels. They are uncommon (<2%).
- -
Neuropathy (intercostal, phrenic, plexo-brachial). Phrenic lesions can reduce the vital capacity up to 1 liter.36 The management of persistent neuropathic pain with gabapentin is effective.20
- -
Implantation in the electrode trajectory or on skin. It is usually detected 3–12 months post-RFA. The ablation of the trajectory reduces the risk.37
- -
Rare complications (<1%):
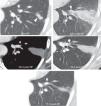
Subcutaneous emphysema (Fig. 13). It disappears spontaneously though sometimes it takes weeks. It can be associated to the pneumo-peritoneum.12 It is more extensive if associated to BPF.
Figure 13.Complications. Pneumothorax (white arrowheads) 24hours after the procedure in 2 patients. Risk factors (RF): long intra-pulmonary trajectory of the radiofrequency needle (black arrowheads), advanced age (89 years the patient above and 70 years the patient below) (controversial RF) and large tumor in the first patient (controversial RF). Subcutaneous emphysema also occurred in the first patient (black arrows).
Air embolism. It occurs when a pulmonary vein communicates with the airways or the atmospheric air.37 If asymptomatic, oxygen at 100% should be administered and the patient positioned in the Trendelenburg or decubitus supine positions.36
Anecdotal (≤0.2%): costal necrosis, pulmonary artery pseudo-aneurism,36 diaphragmatic hernia36 and acute kidney failure.10
D. Clinical.
- -
Productive coughing (0–36%). It can last up to 4 weeks.
- -
Pleuritic chest pain. Common (14–18%). Due to pleural irritation in treated peripheral tumors. I can start after two weeks. It usually lasts for 2–7 days. It can be managed with oral painkillers.
- -
Dyspnea. It might require oxygen therapy.12
- -
Post-ablation syndrome38: fatigue, myalgia and fever. Common. It can last for two weeks. It is attributed to the circulation of toxins from tumor necrosis. It improves with non-steroidal anti-inflammatory drugs.10
- -
Transient FEV1 (Forced Expiratory Volume in the first second) alteration. Tolerance to this modality in terms of respiratory function is generally excellent. Temporary changes can occur with need for oxygen between day 1 and week 3 but not long term.12
- -
Burns caused by the pads. When there are folds in the metal portion of the pads, skin currents are generated that do not allow the even dissipation of heat10 and can cause burns. Their temperature can be monitored.17
- -
The greater frequency of complications in central lesions (64% of lung cancers)17 makes us not recommend the RFA for such lesion.34 Certain nervous lesions are more frequent in apical tumors.39 To insulate the chest wall or the mediastinum from thermal damage in proximal ablations one pleural effusion or pneumothorax can be caused deliberately10,36 by piercing the parietal pleura and letting environmental air into the pleural space.12,19 In hemorrhages surrounding the RFA we should locate the bleeding point with a CT with IV C and treat it immediately.36 Ablation of the trajectory will minimize tumor implantation,30,36 hemorrhages37 and pneumothorax.27 The indication of RFA in radiated patients should be assessed carefully.36 Ventilation with positive pressure favors hemothorax and air embolism though they are very rare.
Major complications (8–12%) are:
- -
Death. In 0.2–2.6%. Mortality rate at 30 days is 3.9% and specific mortality is 2.6%.34 It can occur due to massive pulmonary hemorrhages, respiratory failure, heart failure and myocardial infarction.30 It has been described in patients with pneumonectomy.10,40
- -
Other. Untreatable pneumothorax, air embolism, pulmonary abscesses, >38.5°C fever, massive hemorrhages, pneumonitis, hemothorax, tumor implantation in the trajectory, empyema and skin burns.
It is a priority to research new therapeutic options for lung cancer and pulmonary metastasis. The RFA is a feasible modality. It is crucial to differentiate radiologically normal evolutionary changes from tumor persistence, to be able to know the precautions to minimize their complications–that are not too many but still potentially serious, and to know how to treat them. More research is necessary to define the best follow-up strategy.
Ethical responsibilitiesProtection of people and animalsAuthors declare that for this investigation no experiments on human beings or animals were performed.
Data confidentialityThe authors declare that in this article there are no data from patients.
Right to privacy and informed consentThe authors declare that in this article there are no data from patients.
Conflict of interestsThe author declares no conflict of interests.
Please cite this article as: Plasencia Martínez JM. Radiofrecuencia pulmonar (Parte 2): procedimiento y seguimiento. Radiología. 2015;57:287–302.