To determine the incidence of immune-mediated adverse reactions with and without radiologic manifestations and to correlate them with the response to immunotherapy.
Material and methodsWe retrospectively included 79 patients with stage IV lung carcinomas (n = 24), renal carcinomas (n = 11), or melanoma (n = 44) treated with immunotherapy. We evaluated the occurrence of immune-mediated adverse reactions, their radiologic manifestations, and the response pattern according to the immune-related response criteria (irRC). We correlated the presence of immune-mediated adverse reactions with the response pattern.
ResultsImmune-mediated adverse reactions occurred in 27.8%, being most common in patients with melanoma (40.9%). In 59.1% of patients with adverse reactions, there were radiologic manifestations such as pneumonitis, colitis, hypophysitis, thyroiditis, or myocarditis. Pneumonitis was the most common radiologic manifestation of immune-mediated adverse reactions, even in asymptomatic patients. The rate of response to immunotherapy was higher among patients who developed immune-mediated adverse reactions than in those who did not (68.2% vs. 38.6%, respectively, χ2 5.58; p = 0.018). The rate of favorable responses was higher in patients with radiologic manifestations of immune-mediated adverse reactions than in those without radiologic manifestations (84.6% vs. 44.4%, respectively; p = 0.023).
ConclusionsThe presence of immune-mediated adverse reactions is associated with a better response to immunotherapy. The association with a favorable response is even stronger in patients with radiologic manifestations of the immune-mediated adverse reactions.
Identificar la incidencia de reacciones adversas inmunomediadas (irAE, immune related adverse events), con y sin manifestaciones radiológicas, y correlacionarla con la respuesta al tratamiento inmunoterápico.
Material y métodosSe reclutaron retrospectivamente 79 pacientes con carcinomas de pulmón (n = 24), renal (n = 11) y melanoma (n = 44) en estadio IV que fueron tratados con fármacos inmunoterápicos. Se valoró la aparición de irAE, sus manifestaciones radiológicas y el tipo de patrón de respuesta de acuerdo con los criterios de respuesta a la inmunoterapia (irRC). Se relacionó la presencia de irAE con el patrón de respuesta al tratamiento.
ResultadosEl 27,8% de los pacientes sufrieron irAE. Estas reacciones fueron más frecuentes en pacientes con melanoma (40,9% de los pacientes). Más de la mitad de las reacciones (59,1%) presentaron manifestaciones radiológicas a modo de neumonitis, colitis, hipofisitis, tiroiditis y miocarditis. La neumonitis fue la irAE con expresión radiológica más frecuente, incluso en pacientes asintomáticos. En la población estudiada, la tasa de respuesta a la inmunoterapia fue significativamente mejor en pacientes que desarrollaron irAE (68,2% frente a 38,6%, χ2 = 5,58; p = 0,018). La tasa de respuesta favorable en los pacientes con y sin manifestaciones radiológicas de las irAE fue de 84,6% y 44,4%, respectivamente (p = 0,023).
ConclusionesLa presencia de reacciones adversas inmunomediadas se asocia, de forma significativa, con una mejor respuesta a la inmunoterapia. La asociación con respuesta favorable es incluso mayor en pacientes con manifestaciones radiológicas de las irAE.
In the last decade, immunotherapy has revolutionised the management of patients with advanced tumours and has been put forward as the new paradigm in the treatment of metastatic tumours resistant to first-line treatments.
The journal Science declared immunotherapy as the greatest breakthrough of 2013, basing this statement on the promising results observed in patients with metastatic melanoma treated with ipilimumab.1 This agent, ipilimumab, acts by inhibiting cytotoxic T lymphocyte antigen-4 (CTLA-4), a molecule expressed on the surface of cytotoxic T lymphocytes (TLs).2,3 In normal conditions, CTLA-4 blocks the second co-stimulatory signal of TLs, blocking their activation.
Since its approval in 2011, the success of ipilimumab in the treatment of metastatic melanoma and its good safety profile has led to the development of other immunotherapy agents, such as the receptor inhibitors programmed cell death protein 1 (PD-1) and its ligand, PD-L1.3–5 The binding of PD-1, a molecule expressed on TLs, to its ligand PD-L1 leads to the negative regulation of the immune response, preventing the activation of the cytotoxic TLs. The use of anti-PD-1 drugs, such as nivolumab and pembrolizumab, prevents the inactivation of TLs, so that they can carry out their cytotoxic activity.5
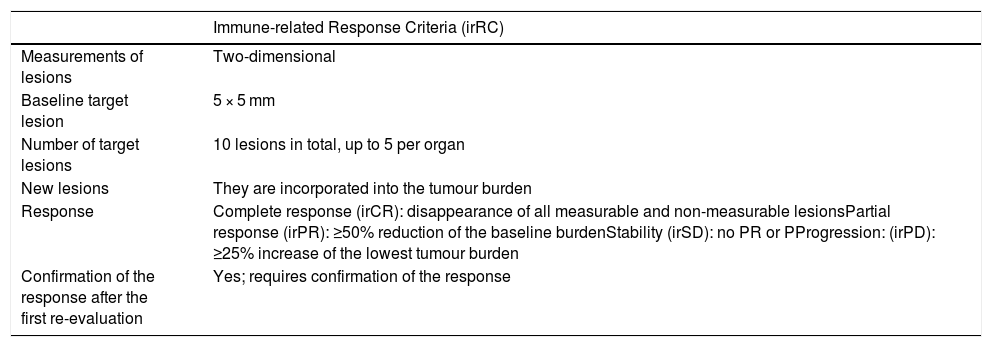
Clinical experience has shown that the response to these agents may be delayed, meaning that the traditional Response Evaluation Criteria in Solid Tumours (RECIST 1.1) are not sufficient to determine the immunotherapy activity.6 This is how the immune-related response criteria (irRC) came about, according to which the onset of new lesions or the initial increase in the size of existing lesions does not necessarily imply treatment failure.7
Furthermore, the activation of the immune system to fight off the tumour can lead to the onset of immune-related adverse events (irAEs).8,9 Several studies suggest that the presence of irAEs predicts a better response to immunotherapy, although there are publications with contradictory data.10,11 Many irAEs, asymptomatic at the outset, can be detected early on by imaging techniques, which enables the corresponding therapy to be started and prevents progression to the most severe degrees of toxicity.
The objective of this study was to identify the incidence of irAEs, with and without radiological manifestations, and to correlate it with the response to immunotherapy.
Material and methodsRetrospective study in which 79 patients with metastatic lung carcinomas (n = 24), renal carcinomas (n = 11) and melanoma (n = 44), who were receiving treatment with immunotherapy drugs, were included (Table 1). These tumours were selected due to their high prevalence and due to having extensive experience with the use of immunotherapy drugs.
Some of the drugs approved by the United States Food and Drug Administration included in the study, with their indications for the tumours included in this publication.
| Drug (target) | Indication |
|---|---|
| Ipilimumab (CTLA-4) | Melanoma |
| Nivolumab (PD-1) | Melanoma, non-small cell lung cancer, renal carcinoma |
| Pembrolizumab (PD-1) | Melanoma, non-small cell lung cancer |
Consecutive patients with at least one imaging control of their underlying condition performed between January and May 2017.
Having available at least two re-evaluations of their disease by imaging tests, four weeks apart.
Patients with synchronous tumours or those who had received treatment as part of a clinical trial with drugs still not approved for these tumour types were excluded. The gender, age, tumour histology and immunotherapy drugs were collected from the recruited patients. There were no patients lost in the recruitment stage.
As it was a retrospective study, approved by the site’s ethics committee, in accordance with current legislation, informed consent of the subjects was not required as no personal interviews or collection of biological samples were carried out.
Imaging techniquesComputed tomography (CT) with intravenous contrast was the most-used imaging test to detect irAEs. All studies were performed in multi-detector equipment (SOMATOM Definition and Sensation, Siemens Healthineers, Germany). To assess specific irAEs, suspected due to symptoms and/or due to laboratory tests, more specific imaging tests such as brain magnetic resonance imaging (MRI) or thyroid ultrasound were performed.
Evaluation of response and recording of irAEsThe response to immunotherapy was evaluated by two radiologists (with 20 and three years of experience, respectively) in chest-abdomen-pelvis CT studies measuring the target lesions of each organ in the last study performed compared to baseline, in accordance with the irRC criteria (Table 2). The response was categorised as favourable or unfavourable, considering as favourable the stability of the lesions, partial response and complete response.
Response criteria for immunotherapy.
| Immune-related Response Criteria (irRC) | |
|---|---|
| Measurements of lesions | Two-dimensional |
| Baseline target lesion | 5 × 5 mm |
| Number of target lesions | 10 lesions in total, up to 5 per organ |
| New lesions | They are incorporated into the tumour burden |
| Response | Complete response (irCR): disappearance of all measurable and non-measurable lesionsPartial response (irPR): ≥50% reduction of the baseline burdenStability (irSD): no PR or PProgression: (irPD): ≥25% increase of the lowest tumour burden |
| Confirmation of the response after the first re-evaluation | Yes; requires confirmation of the response |
irCR: immune-related complete response; irPD: immune-related progressive disease; irPR: immune-related partial response; irSD: immune-related stable disease.
To determine the incidence of irAEs, imaging studies and the medical history since the start of treatment were reviewed. The irAEs included dermatitis, enterocolitis, pneumonitis, thyroiditis, hypophysitis, transaminitis, adrenal insufficiency, pancreatitis, myositis, etc. It was determined which of these reactions presented radiological manifestations.
The following radiological patterns were considered to be manifestations of immune-mediated pneumonitis:
- ◦
Ground-glass opacities, frequently peripheral.
- ◦
Cryptogenic organising pneumonia, manifesting as predominantly peripheral infiltrates, which change location and may exhibit the “reversed halo” sign.
- ◦
Hypersensitivity pneumonitis, especially sub-acute, with centrilobular ground-glass areas and areas of air entrapment.
- ◦
Nonspecific interstitial lung disease, with subpleural lines, peripheral involvement in ground glass, scar tracts, etc.
- ◦
Acute interstitial lung disease. As with other drugs, immunotherapy drugs can cause direct lung toxicity, with risk of acute lung failure and a high rate of mortality.
Immune-mediated thyroiditis and enterocolitis presented nonspecific radiological findings common to other aetiologies.
Statistical analysisA descriptive analysis of the type of response presented by patients in accordance with the irRC was carried out. The absolute and relative frequencies of the irAEs were calculated in the three groups of patients during the time in which they were undergoing treatment with immunotherapeutic agents. The relationship between the type of response (favourable or unfavourable) and the onset of irAEs in the total population was studied using the Pearson’s χ2 test (SPSS 20.0). A p value of <0.05 was considered statistically significant.
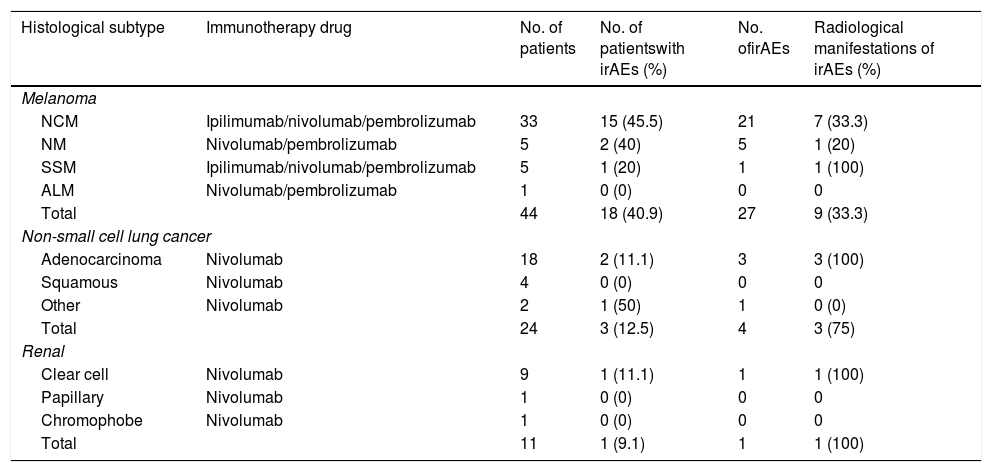
ResultsThe results are summarised in Table 3.
Number of patients with immune-related adverse events in the different tumour groups, depending on the histological type. Those that are accompanied by radiological expression are detailed.
| Histological subtype | Immunotherapy drug | No. of patients | No. of patientswith irAEs (%) | No. ofirAEs | Radiological manifestations of irAEs (%) |
|---|---|---|---|---|---|
| Melanoma | |||||
| NCM | Ipilimumab/nivolumab/pembrolizumab | 33 | 15 (45.5) | 21 | 7 (33.3) |
| NM | Nivolumab/pembrolizumab | 5 | 2 (40) | 5 | 1 (20) |
| SSM | Ipilimumab/nivolumab/pembrolizumab | 5 | 1 (20) | 1 | 1 (100) |
| ALM | Nivolumab/pembrolizumab | 1 | 0 (0) | 0 | 0 |
| Total | 44 | 18 (40.9) | 27 | 9 (33.3) | |
| Non-small cell lung cancer | |||||
| Adenocarcinoma | Nivolumab | 18 | 2 (11.1) | 3 | 3 (100) |
| Squamous | Nivolumab | 4 | 0 (0) | 0 | 0 |
| Other | Nivolumab | 2 | 1 (50) | 1 | 0 (0) |
| Total | 24 | 3 (12.5) | 4 | 3 (75) | |
| Renal | |||||
| Clear cell | Nivolumab | 9 | 1 (11.1) | 1 | 1 (100) |
| Papillary | Nivolumab | 1 | 0 (0) | 0 | 0 |
| Chromophobe | Nivolumab | 1 | 0 (0) | 0 | 0 |
| Total | 11 | 1 (9.1) | 1 | 1 (100) | |
ALM: acral lentiginous melanoma; irAE: immune-related adverse events; irCR: immune-related complete response; irPD: immune-related progressive disease; irSD: immune-related stable disease; irPR: immune-related partial response; NCM: non-classifiable melanoma; NM: nodular melanoma; SSM: superficial spreading melanoma.
The different drugs received according to histology type are included in the group of patients with melanoma (combination therapy or consecutive treatments are not indicated).
Forty-four patients with melanoma, 27 males and 17 females (60.92 ± 12.67 years), who were receiving ipilimumab (n = 20), nivolumab (n = 12), combination therapy of ipilimumab + nivolumab (n = 7) and pembrolizumab (n = 5) were included. The mean follow-up time of the sample studied was 314.6 ± 385.26 days.
Of the 18 patients (40.9%) with melanoma who suffered from irAEs, 12 (66.67%) presented a favourable response (3 irCR [immune-related complete response], 4 irPR [immune-related partial response] and 5 irSD [immune-related stable disease]). A total of 27 irAEs were recorded, nine of which (33.3%) were detected in imaging tests: pneumonitis (n = 5), thyroiditis (n = 2), hypophysitis (n = 1) and colitis (n = 1).
Pneumonitis was presented radiologically as ground-glass infiltrates and peripheral patchy consolidations, some of them with a centre with reduced attenuation (“reversed halo”). Cases of thyroiditis were confirmed on ultrasound, although the diagnosis was clinical and biochemical.
Lung cancerIn the case of patients with lung cancer (n = 24), 15 males and nine females (60.12 ± 11.33 years), who were receiving treatment with nivolumab (mean follow-up of 144.7 ± 122.8 days), were included. Three patients (12.5%) presented four irAEs (nephritis, myocarditis and two cases of pneumonitis), 75% of which had radiological expression. Two of the patients (66.67%) who suffered from irAEs had a favourable response (irSD and irPR).
Kidney cancerThe group of patients with renal carcinoma (n = 11) included eight males and three females (61.9 ± 10.3 years) and they received nivolumab (mean follow-up of 216.36 ± 216.9 days). One of the patients (9%) suffered from myocarditis, which was detected by single-photon emission computed tomography (SPECT) (a cardiac MRI with gadolinium was not performed due to severe kidney failure). The patient was in irSD.
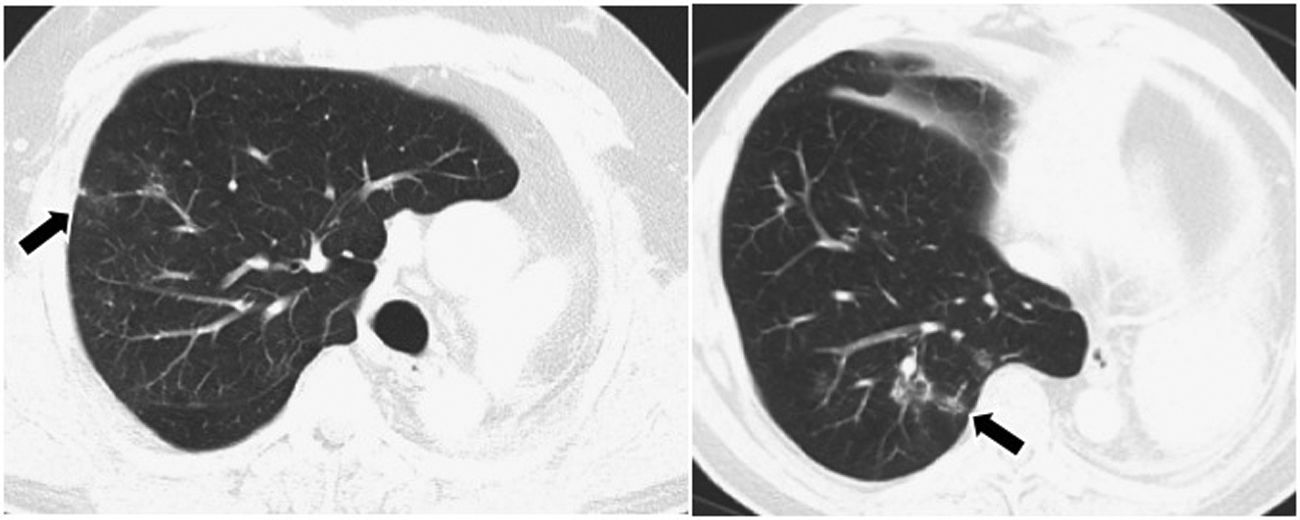
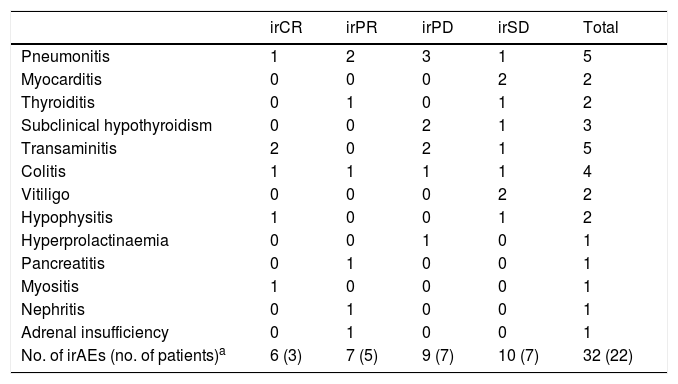
Overall resultsOverall, of the 79 patients, 22 (27.8%) suffered a total of 32 irAEs (Table 4). A total of 59.1% of these reactions presented radiological expression, documented mainly by CT, as pneumonitis, colitis, hypophysitis, thyroiditis and myocarditis (Figs. 1–4). Pneumonitis was the irAE which most often presented radiological expression, even in asymptomatic patients. Radiological patterns of pneumonitis were peripheral ground-glass opacities and infiltrates or consolidations typical of the organising pneumonia pattern.
Number of immune-related adverse events observed in all the groups of patients by response.
| irCR | irPR | irPD | irSD | Total | |
|---|---|---|---|---|---|
| Pneumonitis | 1 | 2 | 3 | 1 | 5 |
| Myocarditis | 0 | 0 | 0 | 2 | 2 |
| Thyroiditis | 0 | 1 | 0 | 1 | 2 |
| Subclinical hypothyroidism | 0 | 0 | 2 | 1 | 3 |
| Transaminitis | 2 | 0 | 2 | 1 | 5 |
| Colitis | 1 | 1 | 1 | 1 | 4 |
| Vitiligo | 0 | 0 | 0 | 2 | 2 |
| Hypophysitis | 1 | 0 | 0 | 1 | 2 |
| Hyperprolactinaemia | 0 | 0 | 1 | 0 | 1 |
| Pancreatitis | 0 | 1 | 0 | 0 | 1 |
| Myositis | 1 | 0 | 0 | 0 | 1 |
| Nephritis | 0 | 1 | 0 | 0 | 1 |
| Adrenal insufficiency | 0 | 1 | 0 | 0 | 1 |
| No. of irAEs (no. of patients)a | 6 (3) | 7 (5) | 9 (7) | 10 (7) | 32 (22) |
irCR: immune-related complete response; irPD: immune-related progressive disease; irPR: immune-related partial response; irSD: immune-related stable disease.
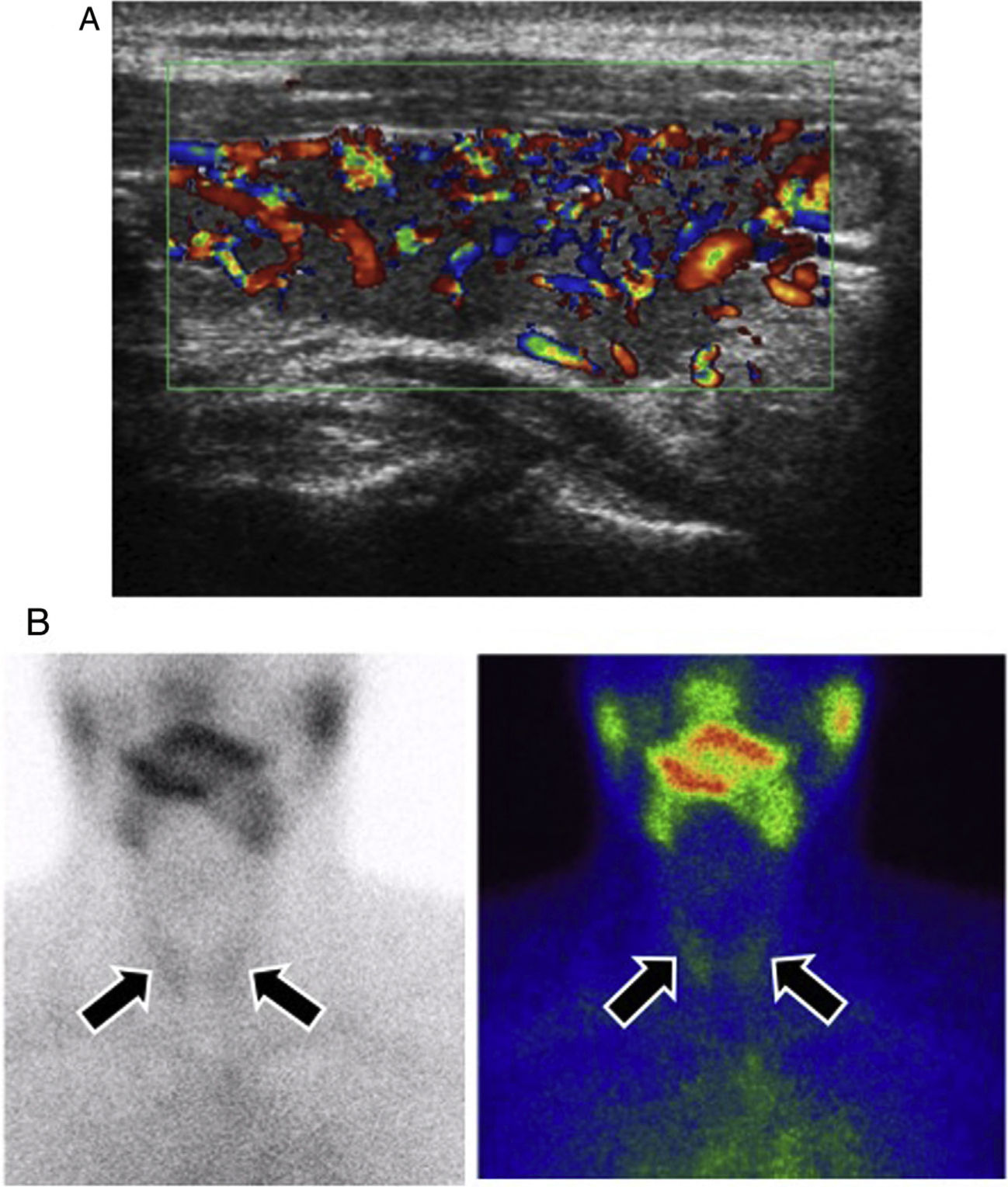
Thyroiditis in a patient with metastatic melanoma undergoing treatment with ipilimumab + nivolumab. A) Colour Doppler ultrasound in the sagittal plane of the right thyroid lobe. Ultrasound signs of thyroiditis, with diffuse vascularisation increase of the thyroid lobe. B) Conventional thyroid scintigraphy in anterior projection with technetium Tc-99 m pertechnetate. High uptake of the radiotracer by both thyroid lobes was confirmed, which is typical of inflammatory processes (arrows).
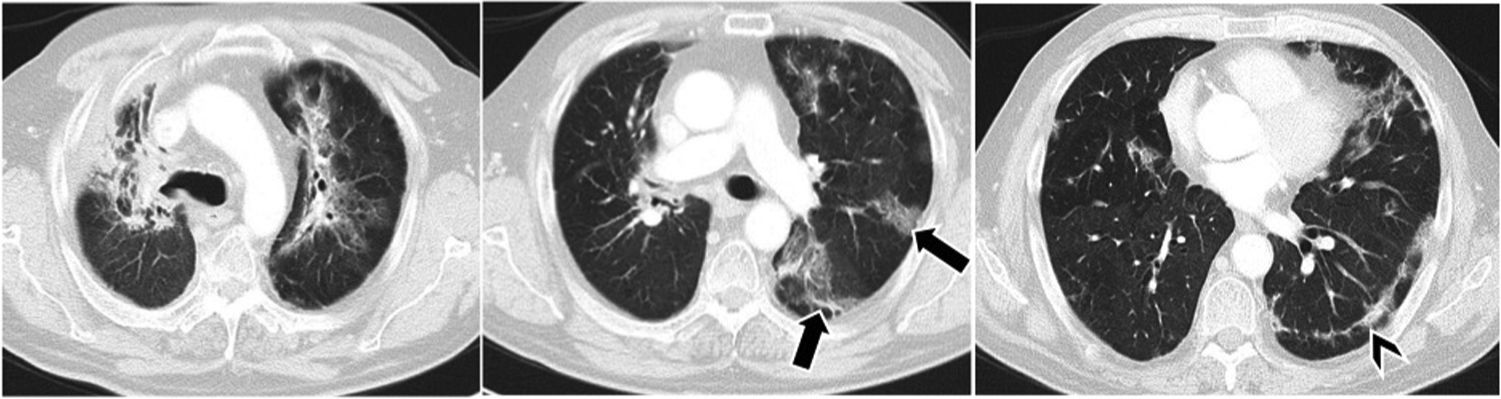
Computed tomography of the chest, axial slices, parenchyma window. Migratory pulmonary infiltrates, of peripheral predominance (arrows), in patient with lung adenocarcinoma undergoing treatment with nivolumab. In baseline slices, subpleural lines (arrowhead) are also seen. Possible immune-mediated pneumonitis, consistent with organising pneumonia.
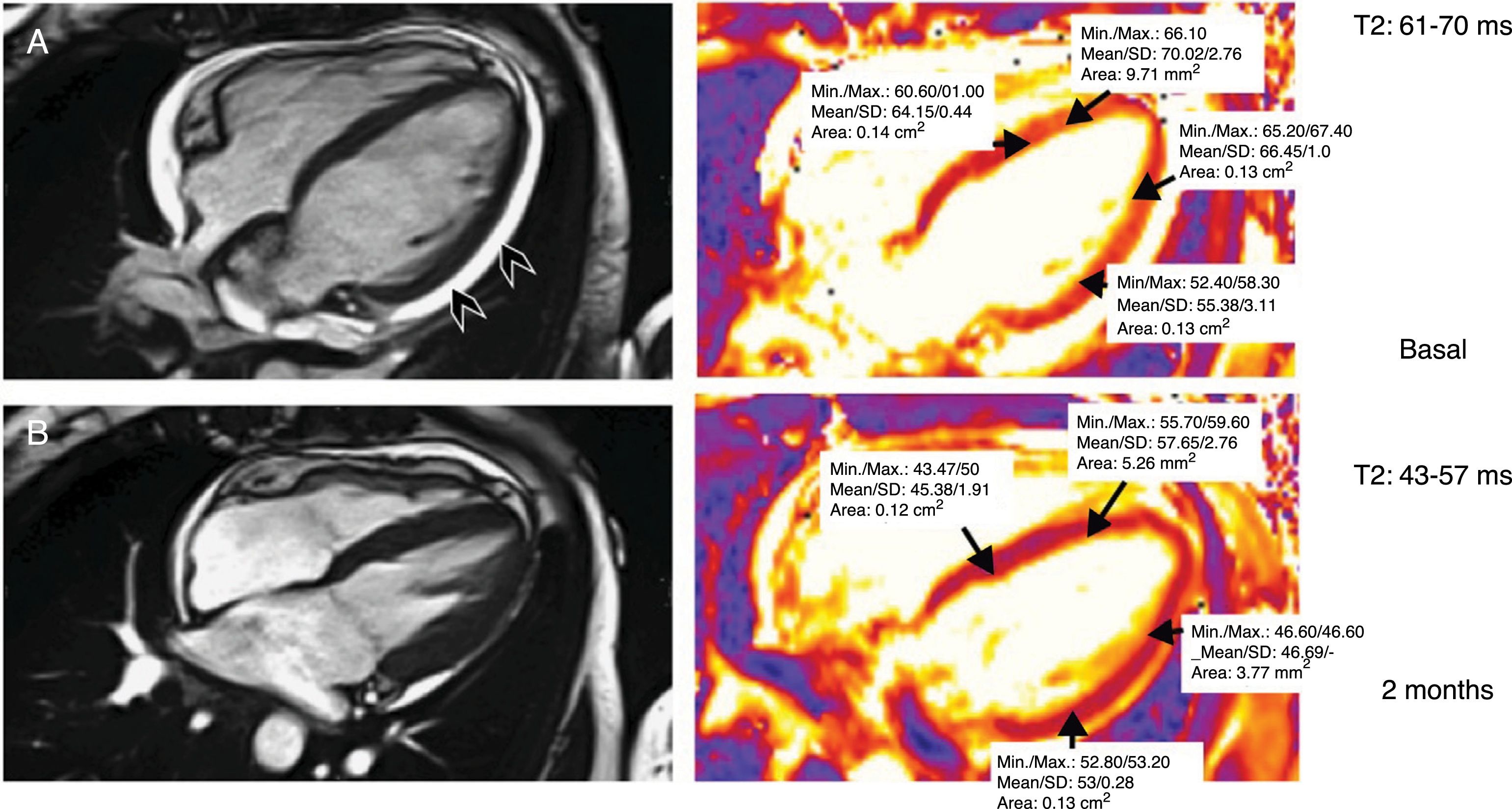
Cardiac magnetic resonance imaging. Gradient-echo sequences using SSFP imaging of the four chambers for anatomical and functional assessment. T2 mapping for quantification of myocardial oedema in a patient with myocarditis induced by nivolumab. Baseline study with increased T2 values due to myocardial oedema and with presence of mild pericardial effusion (arrowheads) (T2 = 61–70 ms). Control performed two months after the episode of myocarditis: resolution of the pericardial effusion and normalisation of the mean T2 values (43–57 ms).
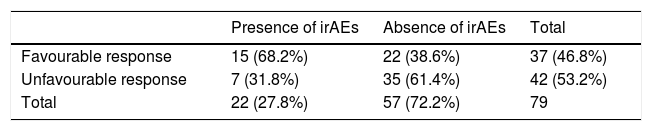
More than two thirds of patients who suffered from irAEs presented a favourable response to treatment, with a significant association being found between both variables (68.2% vs 38.6%, χ2 = 5.58; p = 0.018) (Table 5). The favourable response rate in patients with and without radiological manifestations of irAEs was 84.6% and 44.4%, respectively (χ2 = 5.2; p = 0.023).
Patients from all the tumour groups who had immune-related adverse events, categorised into two immunotherapy response groups.
| Presence of irAEs | Absence of irAEs | Total | |
|---|---|---|---|
| Favourable response | 15 (68.2%) | 22 (38.6%) | 37 (46.8%) |
| Unfavourable response | 7 (31.8%) | 35 (61.4%) | 42 (53.2%) |
| Total | 22 (27.8%) | 57 (72.2%) | 79 |
irAEs: immune-related adverse events.
In the sample studied, 27.8% of the patients had irAEs. Of them, more than half had radiological manifestations of irAEs, in particular pneumonitis. A significant association was found between the onset of irAEs and favourable response to treatment, as well as between the radiological expression of irAEs and favourable response.
irAEs are a unique range of side effects caused by disruption of immune homeostasis.10,12 The mechanism which causes them is still uncertain.4 Several theories have been put forward to explain their onset, such as the increased activity of TLs against antigens of tumour and healthy tissue or the increased levels of proinflammatory cytokines and circulating antibodies. irAEs start during the first weeks (3–12) after the start of treatment, although they may occur at any time, even after finishing therapy.
irAEs can occur irrespective of the tumour type, so it seems that they depend more on the activation of the immune system than on the tumour histology itself. The incidence of irAEs does seem to depend on the type of immunotherapy drug used, and incidences around 60% with ipilimumab and 40% with anti-PD-1 drugs have been reported.10,13,14
irAEs include colitis, dermatitis, pneumonitis and even sarcoid-like reactions. According to published studies, the most common irAEs are skin toxicity (5–16%), gastrointestinal toxicity (8–12%) and pneumonitis (3–6%).10,15–17 The assessment of small biochemical abnormalities is crucial to detect endocrinopathies such as thyroiditis and hypophysitis, elevation of liver enzymes or transaminitis and nephrotoxicity.
In our study, the results obtained are similar to those described in previous publications, although, unlike other cohorts in which skin or gastrointestinal toxicity are predominant, the most common irAE was pneumonitis (n = 7). The second most common toxicity was gastrointestinal (n = 4), in the form of diarrhoea and colitis. In a meta-analysis of studies of patients undergoing treatment with anti-PD-1 drugs, the incidence of pneumonitis was higher in patients with kidney and lung cancer, compared to melanoma.18 In our study, despite the small number of patients, the incidence of pneumonitis was slightly higher in patients with melanoma (11.3%) than in patients with lung cancer (8.3%).
The association between the onset of irAEs and favourable response to treatment is disputed.18,19 A recent retrospective study found no differences in the response of patients with and without irAEs treated with ipilimumab.11 Bronstein et al. published a series of 119 patients with advanced melanoma treated with ipilimumab, 20 of whom (16.8%) suffered from irAEs with radiological expression.2 The authors observed that the disease was controlled in 55% of the patients with radiological manifestations of irAEs, compared to the 10% control rate of the disease in the group without irAEs. In our study, we observed the same phenomenon: there is a significant association between the onset of radiological manifestations of irAEs and disease control compared to irAEs without radiological expression (84.6% versus 44.4% of favourable response, p = 0.023).
Similarly, the rate of favourable response was significantly higher in patients with irAEs, irrespective of whether or not they had radiological expression (68.2% vs 38.6%; p = 0.018). Therefore, the onset of irAEs may indicate that the immune response has initiated and may be an indirect market of response to immunotherapy.
Our study does have several limitations. It is a retrospective study, with a small and heterogeneous number of patients and was performed at only one site, meaning that the results need to be interpreted with caution. Only one descriptive analysis was performed, analysing the incidence of irAEs. It would be interesting to perform studies with more extensive samples of patients, in prospective follow-up, in order to be able to calculate the progression-free interval.
In conclusion, in the sample studied, the presence of irAEs, as well as their radiological expression, are significantly associated with a favourable response to immunotherapy.
AuthorshipResponsible for the integrity of the study: AE, MC; JPDT, AGB, EC, DC, IV and GB.
Study conception: AE and GB.
Study design: AE, AGB, IV and GB.
Data collection: AE, MC, JPDT, AGB, EC, DC and IV.
Data analysis and interpretation: AE, JPDT, AGB, EC, DC, IV and GB.
Statistical processing: AE and AGB.
Literature search: AE, JPDT, AGB, EC and GB.
Drafting of the paper: AE, AGB and GB.
Critical review of the manuscript with intellectually significant contributions: AE, JPDT, EC, DC, IV and GB.
Approval of the final version: AE, MC, JPDT, AGB, EC, DC, IV and GB.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ezponda Casajús A, Calvo Imirizaldu M, de Torres Tajes JP, García-Baizán A, Castañón Álvarez E,Cano Rafart D, et al. Reacciones adversas inmunomediadas como predictoras de respuesta en pacientes oncológicos en tratamiento con inmunoterapia. Radiología. 2020;62:131–138.