We present the case of a 69-year-old man with a history of hypertension, revascularized ischemic heart disease, and double lung transplantation for chronic obstructive pulmonary disease in May 2025, with good lung function at three months and following a standard immunosuppression regimen based on induction with basiliximab and triple maintenance therapy with tacrolimus, mycophenolate, and steroids. The main post-transplant complication has been the development of chronic kidney disease related to the use of calcineurin inhibitors, with baseline creatinine levels around 1.4–1.8mg/dl, highly dependent on tacrolimus levels.
The patient visited his usual healthcare center due to odynophagia and general malaise, and a nasopharyngeal swab was performed, which tested positive for SARS-CoV-2. It was decided to start paxlovid (nirmatrelvir/ritonavir) as antiviral treatment. After 48h, he notified our transplant unit of general malaise, diarrhea, vomiting, and severe tremors. He was instructed to discontinue the drug and come to our hospital for clinical evaluation and testing.
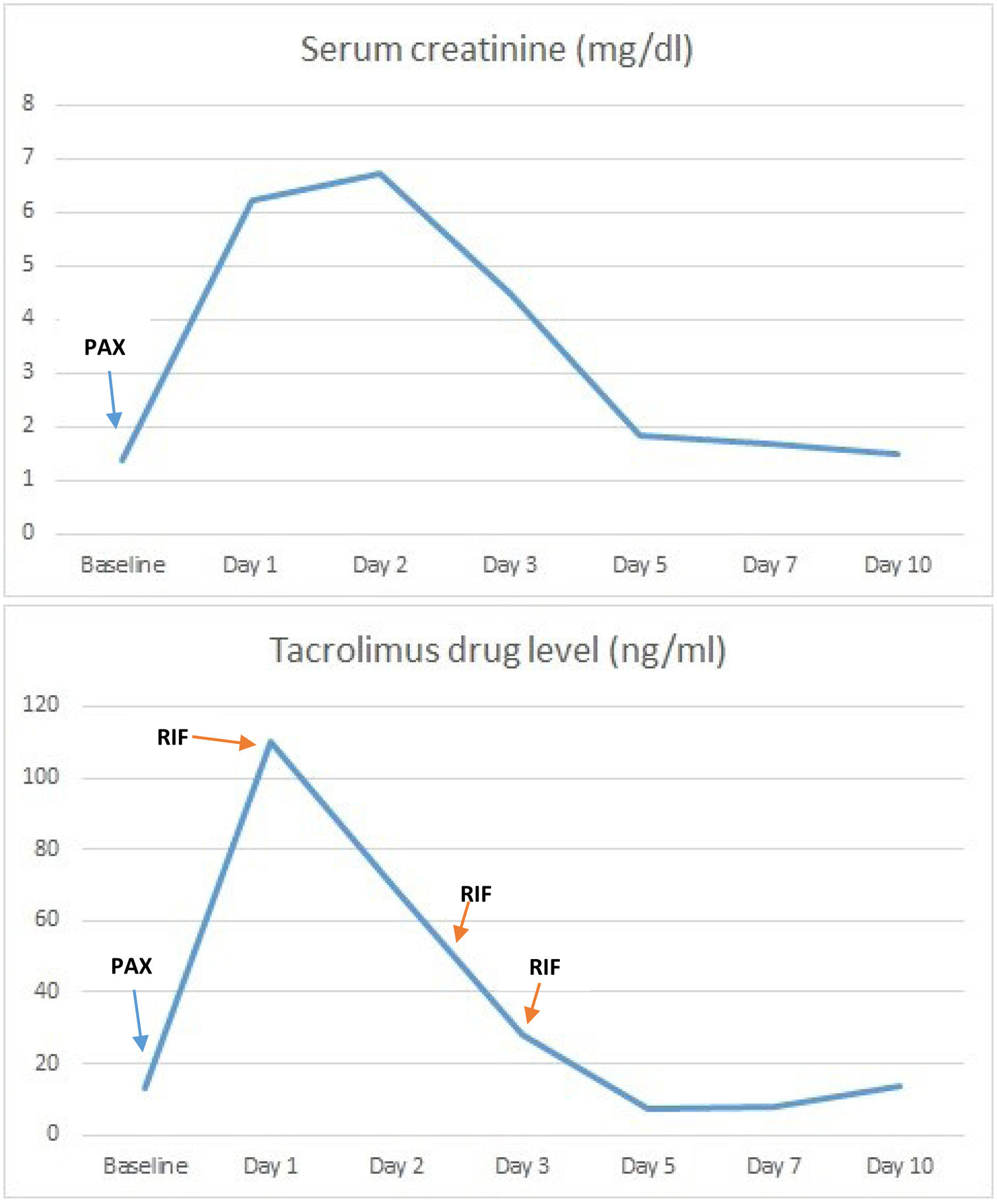
The next day, he comes to our center with the same symptoms. From a respiratory standpoint, he is stable, with a normal X-ray and maximum lung function. Laboratory tests revealed a marked deterioration in renal function with a creatinine level of 6.22mg/dl and metabolic acidosis, along with tacrolimus levels above 160ng/ml (previous levels 18.1ng/ml), being the usual range for the date of transplantation 13–15ng/ml.
It was decided to admit him to hospital to start supportive treatment for renal failure with serum therapy and bicarbonate and to administer rifampicin as a cytochrome P450 inducer. Treatment was initiated with 450mg every 24h, adjusted for renal function according to the drug's technical data sheet. Levels were measured daily, achieving a rapid decrease in tacrolimus levels and a parallel improvement in renal function (Fig. 1). Treatment was discontinued after three doses of rifampicin, when levels of 6.7ng/ml were achieved, at which point tacrolimus was restarted to reach target levels. This also resulted in a marked improvement in the patient's general condition, with the disappearance of tremors and digestive symptoms.
Although the patient presented vomiting and diarrhea at the onset of symptoms, blood pressure remained stable throughout, and upon admission, a urine sodium test was performed, which showed a value of 80mEq/L, ruling out a functional cause for the renal impairment. The patient was discharged from hospital 10 days later, with tacrolimus levels of 13.9ng/ml and recovery of renal function to values similar to his previous ones, with a creatinine level of 1.5mg/dl.
Paxlovid is a combination of the drugs nirmaltrevir and ritonavir that has been used in SARS-CoV-2 infections and may have beneficial effects on mortality and hospitalizations.1,2 Nirmaltrevir is an antiviral that acts as an inhibitor of SARS-CoV-2 3CL protease, an enzyme necessary for viral replication. It is administered together with ritonavir, a potent inhibitor of cytochrome P450 (CYP450), which slows down the metabolism of nirmaltrevir so that it remains active and at a higher concentration for longer. One of the main limitations of the drug's use is its interactions with other drugs that are also metabolized by CYP450, including tacrolimus, which can lead to toxic levels due to this inhibition.
Severe tacrolimus poisoning is a rare but potentially serious condition. It is generally associated with the onset of acute renal failure and neurological toxicity, with a spectrum that can range from mild symptoms, as in our case, to seizures or more severe symptoms.
As for treatment, beyond supportive measures, there is experience in solid organ transplantation with the use of cytochrome P450 inducers,3–5 such as rifampicin or phenytoin, to accelerate tacrolimus metabolism and reduce blood levels of the drug as quickly as possible, with the consequent improvement in neurological symptoms and renal function.
Xiong et al. reported their experience with rifampicin for the treatment of acute tacrolimus poisoning secondary to paxlovid in a lung transplant patient.6 This case shows significant similarities to ours. The patient presents with very similar clinical symptoms to our case, although the symptoms began a little later. The rifampicin dose and the number of administrations required to reduce levels to non-toxic ranges in both patients were exactly the same. However, in this case, the poisoning was less severe as there was no significant renal failure.
The use of CYP450 inhibitors is a useful strategy for the treatment of acute tacrolimus poisoning, although it is not without risks, as it is very difficult to predict how the drug will be metabolized, which can lead to subtherapeutic levels and consequently increase the risk of rejection. Therefore, it should be carefully evaluated and, in our opinion, reserved for the treatment of severe intoxication.
Declaration of generative AI and AI-assisted technologies in the writing processTo write the manuscript we have not use artificial intelligence.
Informed consentInformed consent has been obtained from the patient for the publication of his clinical data.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsFirst author/author for correspondence: Juan Margallo Iribarnegaray.
Co-authors: Lucía Ortega Ruiza, María Ruiz Rodriguez, Rodrigo Alonso Moralejo, Carlos Andrés Quezada Loaiza, Alicia de Pablo Gafas.
All authors approved the final version of the manuscript.
Conflicts of interestThe authors declare not to have any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.