Prevention of exacerbations is a key objective in chronic obstructive pulmonary disease (COPD) management. The adverse effects of an exacerbation include a negative impact on patient quality of life and symptoms, an accelerated rate of decline in lung function, hospital admissions, and increased mortality. Clinical guidelines related to COPD management recommend smoking cessation and inhaled therapy (bronchodilators with or without corticosteroids) as the mainstay for these patients. Apart from the above-mentioned treatment, other potential therapies, such as mucolytic agents, antibiotics (oral or inhaled), phosphodiesterase-4 inhibitors or vaccination, are available and have been shown to reduce the incidence of exacerbations. In this brief narrative review, we will examine the efficacy of various treatments for preventing COPD exacerbations, beyond the use of bronchodilator therapy and inhaled corticosteroids.
La prevención de las exacerbaciones es un objetivo clave en el tratamiento de la enfermedad pulmonar obstructiva crónica (EPOC). Los efectos adversos de una exacerbación incluyen un impacto negativo en la calidad de vida y los síntomas del paciente, un deterioro acelerado de la función pulmonar, un mayor riesgo de ingresos hospitalarios y un aumento de la mortalidad. Las guías de práctica clínica relacionadas con el tratamiento de la EPOC recomiendan el cese tabáquico y la terapia broncodilatadora con o sin corticosteroides inhalados como tratamiento base para estos pacientes. Aparte de los tratamientos mencionados, existen otras terapias potenciales, como son los agentes mucolíticos, los antibióticos (orales o inhalados), los inhibidores de la fosfodiesterasa-4 o la vacunación, entre otros, que han demostrado su papel en la prevención de las agudizaciones. En esta breve revisión narrativa examinaremos la eficacia de estos tratamientos más allá del uso de la terapia broncodilatadora y los corticosteroides inhalados.
Prevention of exacerbations is a key objective in chronic obstructive pulmonary disease (COPD) management.1 Exacerbations have a negative impact on patient quality of life, symptoms and lung function and are associated with a lengthy recovery, an accelerated rate of lung function decline and more hospital admissions.1–3 They are also related with significant mortality, particularly in cases that require hospitalization, and are associated with a high socioeconomic burden, as these events account for most of the total COPD healthcare expenditure.1–3
Smoking cessation is the most effective initial strategy: it reduces COPD progression, particularly the risk of exacerbation and lung function, and significantly decreases the risk of mortality.1,4,5 Nevertheless, a significant number of COPD patients continue to smoke.1,4,5
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) provides treatment recommendations for patients who are at high risk of exacerbation. The primary treatment options recommended for these patients are a long-acting muscarinic antagonist (LAMA), alone or in combination with a long-acting β2-agonist (LABA). Inhaled corticosteroids are recommended for patients at high risk of exacerbation who experience further exacerbations following initial bronchodilator treatment and show an elevated peripheral blood eosinophil count.1 Apart from the above-mentioned molecules, other potential therapies such as mucolytic agents, antibiotics, phosphodiesterase-4 inhibitors (PDE4i), or vaccination, among others, are available, and have been shown to reduce the risk of exacerbation.1
In this brief narrative review, we will examine the efficacy of various treatments for preventing COPD exacerbations, beyond the use of bronchodilator therapy and inhaled corticosteroids.
N-acetylcysteineN-acetylcysteine (NAC) was approved as a prescription medication by the Food and Drug Administration in 1963. It acts at several molecular levels, showing, as follows: mucolytic properties, breaking disulfide bonds in high-molecular-weight glycoproteins within mucus; anti-inflammatory properties, suppressing the activation of the nuclear factor-kB transcription factor and subsequent cytokine production; antibiofilm activity and bacterial adherence inhibition; and direct antioxidant effects on certain oxidants while increasing intracellular cysteine levels, thereby enhancing the synthesis of the antioxidant glutathione.6 These mechanisms may be beneficial for the treatment and prevention of COPD exacerbations.
Several randomized clinical trials (RCTs) with varying recruitment criteria, study durations, and NAC dosages have assessed the potential role of this orally administered drug in preventing COPD exacerbations, yielding somewhat conflicting results. The largest RCTs to date are two Chinese studies. The first, the PANTHEON study which randomized 1006 patients with moderate-to-severe COPD and followed them for one year, demonstrated that treatment with NAC 600mg twice daily was associated with a reduction in exacerbations compared to placebo.7 In contrast, a recent study by Zhou et al.8 that recruited 968 patients, of whom 924 patients completed at least one follow-up visit and 656 a two-year follow-up, found that the same NAC dosage had no significant effect on the primary outcome of annual total exacerbation rates. This study included patients with less severe (mild-to-moderate) COPD than the PANTHEON study (mean FEV1 78% vs. 49% in PANTHEON), suggesting that NAC may be less beneficial for milder cases of COPD. Nonetheless, it is worth noting that, in the Zhou et al. study, the annual rate of moderate or severe exacerbations was lower in the NAC group,8 indicating a potential benefit of NAC for preventing more clinically significant exacerbations.
Multiple systematic reviews and meta-analyses have evaluated the impact of chronic NAC treatment on COPD and most have found a reduced likelihood of exacerbations.9–11 The effect appears more consistent among patients with spirometry-confirmed COPD receiving higher NAC doses (e.g., 600mg twice daily).9 While some studies indicate that the risk reduction for exacerbations with NAC is primarily observed in patients not receiving inhaled steroids,12,13 a Cochrane review concluded that concurrent inhaled steroid treatment does not significantly modify the effect of NAC on exacerbations.11 Notably, older studies tend to report greater benefits, possibly reflecting improvements in pharmacological maintenance therapy for COPD over the past decades that may make it challenging to detect additional benefits from NAC. However, since the 2000s, this trend could also be influenced by efforts to reduce publication bias by encouraging the publication of negative results.11
NAC is generally well-tolerated at the doses used in RCTs, with mild adverse effects mainly limited to gastrointestinal symptoms. These adverse effects do not significantly differ from those in the placebo group, and the risk of side effects does not appear to be dose-dependent when comparing lower and higher NAC doses (e.g., 600 vs. 1200mg/day).9,11 Therefore, the risk–benefit ratio appears favorable for NAC in patients with COPD who experience frequent exacerbations despite adequate inhaled maintenance therapy.
ProbioticsCOPD is characterized by significant chronic inflammation and airway remodeling. The gut microbiota regulates not only digestion and intestinal metabolism but also modulates innate immunity and maintains intestinal barrier function and intestinal barrier homeostasis. The bidirectional interaction between the intestinal microbiome and the lung is known as the gut–lung axis.14 Several studies have shown that in COPD patients there is an alteration (dysbiosis) of the intestinal microbiota.15 This results in an increase in potentially pathogenic bacteria and oxygen free radicals in the intestinal epithelium and an increase in mucosal permeability. All these factors favor the migration of intestinal bacteria and endotoxins to other organs, such as the lung and intestinal lymph nodes, increasing proinflammatory mediators and decreasing the presence of some metabolites beneficial to the organism, such as short-chain fatty acids, thus further aggravating the disease.16,17 Correction of intestinal dysbiosis using probiotics,18,19 prebiotics, synbiotics, a fiber-rich diet, or fecal microbiota transplantation may have beneficial effects on the pathogenesis of COPD.19
Probiotics are live microorganisms that when administered in adequate amounts are beneficial to host health. They modify the intestinal microbiota and can activate the immune system, reducing serum levels of proinflammatory cytokines and increasing anti-inflammatory cytokines. They also hinder bacterial translocation and promote the integrity of the intestinal mucosal barrier, which may help break the vicious circle of gut–lung inflammation. Another way in which they may attenuate the progression of COPD is by decreasing oxidative stress caused by oxygen free radicals produced in the lung in response to tobacco smoke. These free radicals reach the gastrointestinal tract via the bloodstream, exacerbating damage in the gut and subsequently in the lung via the gut–lung axis.20
A recent meta-analysis on the role of probiotics in COPD analyzed three randomized, double-blind, placebo-controlled studies in humans and five randomized controlled studies in animals.19 The large heterogeneity among the studies prevented robust conclusions. Probiotics showed a positive effect on COPD in three ways: (a) they significantly increased FEV1 (%) in people with stable-phase COPD; (b) they significantly decreased inflammation in COPD (increased IL-10 and decreased TNF-α, IL-1β and IL-6), without modifying C-reactive protein in either patients or animals; (c) they significantly reduced lung deposition of collagen fibers in animals with COPD. Furthermore, we should remember that probiotics, by decreasing the frequency of upper respiratory tract infections,21 may reduce the number and severity of exacerbations caused by infections in COPD patients.
In conclusion, although further studies are needed to confirm this hypothesis, probiotics, by acting on the gut–lung axis, may be beneficial in the treatment of COPD patients.
Oral antidiabeticsExtrapulmonary comorbidities are important conditions that contribute to the risk of exacerbations. Type 2 diabetes mellitus (T2DM), a disease commonly found in patients with COPD, is particularly relevant in this context.22 Increased glucose concentrations impair polymorphonuclear leukocyte function, decreasing their phagocytic function and altering host defense. As a consequence, infections are more frequent in patients with T2DM, especially in those with poorer glycemic control.23 Likewise, COPD patients, regardless of the presence of diabetes mellitus, show a higher concentration of glucose in the airway compared with smokers without COPD, and this phenomenon has been associated with increased exacerbations.24
In addition to their favorable metabolic and cardiovascular effects, hypoglycemic drugs also have a potentially positive impact at the respiratory level. Metformin has been shown to have anti-inflammatory and antioxidant effects and prevents airway smooth muscle proliferation through inhibition of AMPK-mediated TGF-β1 signaling. Studies conducted in allergic and obese asthma models have shown that metformin reduces eosinophilic airway inflammation.25 Thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists also produce anti-inflammatory effects and may reduce airway hyperresponsiveness.26 Moreover, in studies conducted in animal models, drugs such as metformin and dapagliflozin (SGLT-2 inhibitors) reduced glucose concentrations in the respiratory tract and inhibited the growth of Staphylococcus aureus or Pseudomonas aeruginosa.
Based on the above, there is a growing interest in determining whether the use of oral antidiabetics can provide any benefit in the prevention of exacerbations. Metformin has been associated with a reduction in exacerbations in diabetic subjects with asthma–COPD overlap.27 A population-based cohort study carried out by Pradhan et al.28 using an active comparator concluded that GLP-1 receptor agonists and SGLT-2 inhibitors, have been associated with a significant reduction in the risk of exacerbations in patients with both COPD and T2DM compared to sulfonylureas. In contrast, DPP-4 inhibitors produced only a modest and non-significant reduction in the risk of exacerbations. Foer et al.29 examined associations between GLP-1 receptor agonists use and risk of exacerbations in COPD patients with T2DM compared with other T2DM medications. The risk of moderate and severe exacerbations was lower with GLP-1 receptor agonists compared to sulfonylureas and DPP-4i, but similar to the risk with SGLT-2 inhibitors, after accounting for recent exacerbation history and the effects of these drugs on metabolic variables (body mass index or baseline glucose control). Moreover, Shanmugavel Geetha et al.30 also observed that in patients with T2DM and COPD, SGLT-2 inhibitors were associated with fewer emergency room visits.
Considering the evidence available to date, oral antidiabetic agents show promise in reducing exacerbations among COPD patients with T2DM. Several studies suggest that these patients may benefit from the use of GLP-1 receptor agonists and SGLT-2 inhibitors compared to sulfonylureas and DPP-4i, as they appear to reduce the risk of exacerbations. However, since most analyses are observational and based on electronic medical records, properly designed studies are needed to confirm the possible role of these drugs in this group of patients.
Monoclonal antibodiesEffective therapeutic options in COPD have the effect of relieving dyspnea and reducing exacerbations. Various drugs and interventions have been studied in this field but, except for smoking cessation, none have been shown to substantially change the natural history of the disease.1 This will only be achieved with therapeutic alternatives that modify the pathogenic mechanisms that maintain the development of airway remodeling and slow the progression of pulmonary emphysema. Dupilumab, the first biologic for COPD, has been approved in the United States, the European Union and China, and we are now on the verge of a new therapeutic era that will modify the course of this disease.31
Over the last few years, the potential benefit of certain monoclonal antibodies in reducing COPD exacerbations has been explored. Research has focused on patients with the so-called “T2 inflammatory phenotype”, i.e. patients with elevated levels of eosinophils in peripheral blood. It is hypothesized that, as in non-allergic eosinophilic asthma, blocking mediators involved in T2 inflammation would result in a reduction of exacerbations. Trials have focused on patients with COPD on triple inhaled therapy, a previous history of exacerbations and an elevated eosinophilic profile.
Firstly, the role of mepolizumab, a monoclonal antibody against interleukin 5 (IL-5), was initially evaluated in two clinical trials with two different doses of the drug vs. placebo (100mg in METREX and 100mg or 300mg in METREO).32 In METREX, but not in METREO, there was evidence of a reduction in the annual rate of moderate and severe exacerbations. The ongoing phase 3 MATINEE trial is expected to provide further data on the use of this drug in COPD patients with an eosinophilic phenotype.
Secondly, Criner et al.33 analyzed the effect of benralizumab (a monoclonal antibody against IL-5 receptor α) vs. placebo in COPD patients with a previous history of exacerbations. Two parallel clinical trials (TERRANOVA and GALATHEA) were also conducted with different drug doses using a slightly higher eosinophil cutoff point than in previously discussed clinical trials. In these trials, benralizumab did not reduce the annual rate of exacerbations despite a drastic reduction in blood eosinophils.
Recently, two new clinical trials (BOREAS and NOTUS) led by Bhat et al.34,35 have been published evaluating a third monoclonal antibody, dupilumab, which inhibits both IL-4 and IL-13 signaling through blockade of a key common receptor in T2 inflammation. The patient profile included subjects with COPD, chronic bronchitis, prior history of two or more moderate exacerbations or one severe exacerbation in the previous year despite triple inhaled therapy, and an eosinophil count >300cels/μL. Compared to placebo, a biweekly dose of dupilumab 100mg reduced the annual rate of exacerbations and improved lung function and quality of life throughout the 52-week follow-up of the study. In light of these findings, the 2025 GOLD document1 has already incorporated the recommendation that dupilumab be considered as a treatment in the follow-up of COPD patients with a history of exacerbations despite triple inhaled therapy, chronic bronchitis, and an elevated eosinophil count.
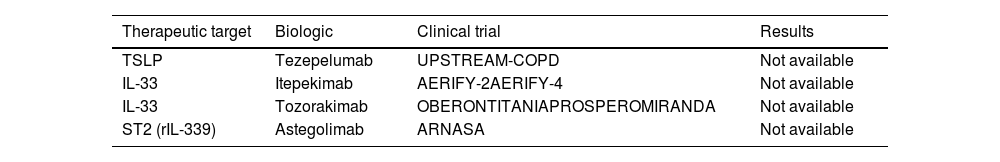
Finally, several ongoing clinical trials are studying biologic drugs that block other therapeutic targets such as TSLP (thymic stromal lymphopoietin), IL-33 and ST2, although results are not available to date (Table 1).36,37 However, the results of the COURSE trial evaluating the efficacy and safety of tezepelumab, a monoclonal antibody that blocks thymic stromal lymphopoietin activity, are now available. This study failed to achieve its primary endpoint of reducing the annual exacerbation rate over 52 weeks of follow-up.38
A recent systemic review was carried out by Pitre et al.39 to analyze the benefits of the different biologics studied to date in COPD patients. Dupilumab reduced moderate-to-severe exacerbations in COPD with elevated blood eosinophils, unlike other biological therapies, including asteogolizumab, benralizumab, mepolizumab, itepekimab or tezepelumab, none of which had any significant effect on patient-relevant outcomes.
Based on the above and taking into account the benefits obtained by these treatments in the field of asthma, practitioners are naturally eager to know what their impact will be on COPD patients. With studies in favor of the use of dupilumab in exacerbating COPD patients with an eosinophilic profile, further analysis is needed to assess the impact of this treatment and to define a more accurate profile of patients who are candidates for treatment with monoclonal antibodies.
VaccinationPatients with COPD are at risk of exacerbations41 which are associated with significant morbidity and mortality and impaired quality of life.42 Most exacerbations are triggered by viral and/or bacterial infections.42 For this reason, COPD patients should receive vaccination recommendations according to applicable local guidelines (Table 2).1
Recommended vaccines in COPD patients (modified reference 1).
| Level of evidence | |
|---|---|
| Influenza vaccine | B |
| Pneumococcal vaccine | B |
| Respiratory syncytial virus vaccine | A |
| Tdap vaccine (tetanus, diphtheria, pertussis)* | B |
| Herpes zoster vaccine | B |
| SARS-CoV-2 vaccine | B |
Seasonal influenza vaccination is recommended for all COPD patients as it decreases the risk of exacerbations, hospitalization for lower respiratory tract infection, and mortality.1,42 In addition, it has been suggested that COPD patients, especially the elderly, have a lower risk of coronary ischemic events when vaccinated against influenza. Vaccines containing killed or inactivated live viruses are recommended, as they are more effective in older people with COPD.1
Pneumococcal vaccination is indicated in patients aged ≥65 years. It is also recommended in adults aged 19–64 years if they have underlying diseases.1 Four vaccines are currently available, the 23-valent pneumococcal polysaccharide vaccine (PPV23) and three conjugates with 13 (PCV13), 15 (PCV15) and 20 serotypes (PCV20).40 Data on the effects of pneumococcal vaccination in patients with COPD are limited.43 Comparative studies in COPD patients have shown that PCV13 is more effective and has the same or higher sustained immunogenicity than PPV23. It also reduces exacerbations and prevents community-acquired pneumonia caused by vaccine serotypes and invasive pneumococcal disease in adults ≥65 years of age.44 PCV20 has demonstrated strong immunogenicity and is currently the vaccine of choice over the previous vaccines.45
Respiratory syncytial virus (RSV) can have important consequences in people aged ≥60 years, particularly those with chronic conditions such as COPD.46 Vaccination against RSV effectively reduces the risk of disease and decreases its severity and is therefore recommended for these patients.1
Patients with COPD are at higher risk of developing pertussis, with a poor outcome and hospitalization. Therefore, administration of the Tdap vaccine (tetanus, diphtheria, pertussis) is recommended in individuals who were not vaccinated in adolescence, to provide protection against this disease, in addition to tetanus and diphtheria.
COPD increases the risk of herpes zoster infection and its complications, and this risk is even higher in patients receiving inhaled corticosteroids. Routine use of the herpes zoster vaccine is now recommended in COPD patients aged ≥50 years.1
Finally, people with COPD should receive the COVID-19 vaccine according to national recommendations. These vaccines are highly effective against SARS-CoV-2 infection requiring emergency care, hospitalization, or intensive care unit admission, including people with chronic respiratory disease.1
In view of the above, vaccination is considered one of the most effective preventive measures in modern medicine. In chronic diseases such as COPD, vaccination is an mainstay in the prevention of respiratory infections.
Oral antibiotics (macrolides and tetracyclines)In the area of antibiotics, several studies have explored the use of azithromycin as prophylactic therapy to reduce the frequency of exacerbations and improve quality of life in patients with COPD.
Azithromycin, a macrolide with antimicrobial, anti-inflammatory and immunomodulatory properties, has been shown to be effective in reducing exacerbations in patients with a history of moderate and severe exacerbations in the previous 12 months, especially when used in addition to standard inhaled therapies. The largest clinical trial, conducted by Albert et al.,47 demonstrated that a daily dose of azithromycin (250mg) significantly reduced the frequency of exacerbations (HR 0.73; CI95% 0.63–0.84, p<0.001), increased time to first exacerbation compared to placebo (266 vs. 174 days, respectively; p=0.001), and improved quality of life.
Subsequently, numerous studies have supported this protective effect of macrolides against exacerbations. A meta-analysis including 14 studies and almost 4000 patients showed that continuous or intermittent use of macrolides compared to placebo resulted in a 14% decrease in the number of patients with one or more exacerbations.48 In another systematic review,49 including nine RCTs and almost 2000 patients, long-term use of macrolides reduced exacerbations (OR 0.34; CI95%: 0.19–0.59, p<0.001). This effect was more significant in 12-month regimens (OR 0.27; CI95%: 0.11–0.68, p=0.005). However, improvement in quality of life as measured by the St. George's Respiratory Questionnaire did not reach clinical significance.
Recent cost-effectiveness analyses reinforce the clinical utility of azithromycin. In 2023, a study concluded that azithromycin is cost-effective in exacerbating patients, with an incremental cost saving per quality-adjusted life year (QALY) of about €6000.50
However, the potential adverse effects of macrolides should not be overlooked. Of particular note is ototoxicity with reversible or partially reversible hearing loss, and QT interval prolongation. This prolongation has been associated with an increase in cardiovascular events but no change in cardiac mortality.48 Furthermore, several meta-analyses have shown that chronic treatment with macrolides increases antimicrobial resistance rates, particularly in pathogens such as Haemophilus influenzae or P. aeruginosa,49 and respiratory mycobacterial infection must be ruled out prior to use.
When comparing antibiotics,51 macrolides, specifically azithromycin, have been shown to significantly reduce COPD exacerbations [HR 0.67; CI95%: 0.69–0.75] compared to quinolones or tetracyclines, which failed to demonstrate a statistically beneficial effect in the included studies.
Results to date for tetracyclines, specifically doxycycline, have been mixed. In several comparative studies with placebo, doxycycline has not been shown to reduce exacerbation rates or improve inflammatory markers despite its anti-inflammatory and antibiotic properties.52 However, recent studies suggest that its potential benefit may be limited to a subgroup of patients. Allinson et al.53 examined the impact of doxycycline use in COPD patients with frequent exacerbations. While doxycycline did not significantly reduce the overall rate of exacerbations in patients during the 12-month follow-up, it did appear to show benefit in those with more advanced obstruction [RR 0.36; CI95%: 0.15–0.85; p=0.019] or in those with peripheral blood eosinophil counts below 300cells/μL [RR 0.50; CI95%: 0.29–0.84; p=0.01]. While these results suggest a potential benefit of doxycycline, further studies are required to validate these findings in specific subgroups.54
In conclusion, long-term use of azithromycin or erythromycin suppresses COPD exacerbations, and previous studies have supported the advantages of a 12-month macrolide prescription over a placebo. Patients categorized as GOLD group E could potentially derive benefits from macrolide prescriptions. Pending further study, doxycycline could be a possible alternative in COPD patients with frequent exacerbations with low peripheral blood eosinophil counts.
Inhaled antibioticsInhaled antibiotic therapy is a very attractive therapeutic option in the treatment of pulmonary infections because of the high concentration of the antimicrobial agent that is achieved at the focus of infection. In the case of COPD, the presence of chronic bronchial inflammation favors the isolation of potentially pathogenic microorganisms in cultures of respiratory samples,55 and these findings are more frequent in more severe patients, frequent exacerbators, and patients with bronchiectasis.56
The presence of pathogenic microorganisms during the stable clinical phase of COPD leads to worse outcomes and more frequent and severe exacerbations.57 When the criteria for chronic bronchial infection are met, the development and/or progression of bronchiectasis is more likely. This defines a specific infectious phenotype of COPD characterized by increased symptomatology, more frequent and severe exacerbations, and a higher risk of mortality.55–57 In these patients, inhaled antibiotic treatment is key because it reduces bacterial counts, reduces exacerbations and improves lung function.58Table 3 shows the available inhaled antibiotics.
Main inhaled antibiotics available.
| - Colistimethate sodium (dry powder or solution for inhalation) |
| - Aztreonam lysine (solution for inhalation) |
| - Tobramycin (dry powder or solution for inhalation) |
| - Gentamicin (intravenous formulation for inhalation) |
| - Levofloxacin (solution for inhalation) |
Although the formal indication for all antibiotics specifically formulated for inhalation is chronic bronchial infection by P. aeruginosa in patients with cystic fibrosis, they are being slowly incorporated into the therapeutic arsenal for patients with chronic bronchial infection associated with non-cystic fibrosis bronchiectasis, including COPD patients with an infectious phenotype.59 The results obtained to date in this group of patients are based mostly on studies with small sample sizes.60–62 There are currently no published multicenter clinical trials specifically examining the direct effects of inhaled antibiotics in COPD patients. One of the most relevant studies has been performed by de la Rosa et al.59 who analyzed the impact of the use of inhaled antibiotics in more than 600 COPD patients with frequent exacerbations (largely with isolation of P. aeruginosa in respiratory samples). The authors observed that after one year of treatment with inhaled antibiotics, there was a significant decrease in the number of exacerbations, hospital admissions and days of hospitalization regardless of the presence of bronchiectasis. Another study examined whether the introduction of nebulized colistimethate sodium in COPD patients with P. aeruginosa infection was associated with a decrease in exacerbations. The authors concluded, after analyzing of a sample of 36 patients, that there was a significant decrease in the number and duration of hospitalizations for exacerbations.60 In another uncontrolled study conducted in patients with severe COPD and colonization with multidrug-resistant P. aeruginosa, an inhaled tobramycin solution 300mg twice daily for 14 days was administered, showing a 42% reduction in the incidence of acute exacerbations at 6 months post-treatment, compared to the 6 months pre-treatment.61 Apart from colistimethate sodium, other specific antibiotic formulations for inhalation used in this group of patients include tobramycin, aztreonam lysine or levofloxacin.62
In view of the above, inhaled antibiotics seem to be effective in reducing the number and severity of exacerbations in patients with an exacerbator or infectious profile regardless of the presence of bronchiectasis, particularly in individuals with chronic bronchial infection caused by P. aeruginosa.
Phosphodiesterase inhibitorsPhosphodiesterase (PDE) enzymes play a key role in the degradation of cyclic AMP (cAMP) and cyclic GMP (cGMP), key second messengers in several signaling pathways whose presence modulates cellular inflammation. At the pulmonary level, PDE 3, 4, 5 and 7 are essential in the regulation of smooth muscle tone, inflammation and immune response, making them key therapeutic targets in COPD.
Roflumilast, a selective PDE4 inhibitor, was approved more than a decade ago as a last-line therapy in the treatment of COPD,63 being the only phosphodiesterase inhibitor indicated in COPD treatment guidelines for patients with severe and very severe airflow obstruction (FEV1<50%), presence of chronic bronchitis, and frequent exacerbations.1 Its mechanism of action is based on the reduction of inflammation, decreasing the infiltration of inflammatory cells and the release of proinflammatory mediators. A recent Cochrane systematic review reported a 12% reduction in the overall rate of exacerbations and a decrease in the percentage of patients with exacerbations from 33% to 27%. Furthermore, severe exacerbations were reduced by 8.5% to 24.3%, with a greater impact in patients with a history of hospitalization for acute exacerbations.64,65 However, its use is limited by its side effects, mainly gastrointestinal (diarrhea, nausea, weight loss) and psychiatric disorders (anxiety, insomnia, depression), which can affect adherence to treatment. Garbe et al.66 have shown that it has an acceptable long-term safety profile and a greater benefit in reducing the risk of mortality in patients with more severe disease.
Ensifenthrine, a selective dual inhibitor of PDE3 and PDE4, has recently been shown to reduce the rate of moderate-to-severe exacerbations and to prolong the time to first exacerbation in COPD patients with moderate-to-severe obstruction, according to the ENHANCE-1 and ENHANCE-2 clinical trials.67 Its route of administration via inhalation favors a more tolerable safety profile, with an adverse event rate similar to that of the placebo group. It is currently approved for use only in the United States. A pooled analysis of both phase 3 trials showed that treatment with ensifenthrine for 24 weeks was associated with a 41% reduction in both the rate of moderate-to-severe exacerbations and the risk of first exacerbation, positioning it as a promising therapeutic alternative in COPD.68
There are other alternatives such as tanimilast, a selective PDE4 inhibitor administered by the inhaled route, which demonstrated promising results in a phase 2 clinical trial.69 Further clinical trials are now being developed to confirm its efficacy and safety in the reduction of exacerbations in COPD patients (https://clinicaltrials.eu).
ConclusionThis review highlights various therapeutic strategies beyond conventional bronchodilator therapy and inhaled corticosteroids for preventing exacerbations in COPD. Monoclonal antibodies, particularly dupilumab, have been seen to be effective in patients with eosinophilic COPD, while vaccination remains a cornerstone of preventive care against respiratory infections. Oral antibiotics have been linked to a reduction in exacerbations, although concerns about antimicrobial resistance persist. Inhaled antibiotics present an attractive therapeutic option, especially for patients with chronic P. aeruginosa bronchial infections. Phosphodiesterase inhibitors have also proven effective in reducing the risk of exacerbations, while some studies suggest that NAC may help lower exacerbation rates, particularly in patients with moderate-to-severe COPD. Probiotics may also contribute by alleviating inflammation through the gut–lung axis. Finally, oral antidiabetic agents show promise in reducing exacerbations among COPD patients with T2DM. Overall, these findings support a multifaceted approach to preventing COPD exacerbations, highlighting the need for further research to optimize treatment regimens.
Declaration of generative AI and AI-assisted technologies in the writing processThis study was developed without the use of any artificial intelligence software or tool.
FundingThis research was supported by SEPAR (Sociedad Española de Neumología y Cirugía Torácica) COPD working group.
Authors’ contributionsAll authors were involved in study conception and design, data acquisition, analysis, interpretation, and drafting and revising the article critically for important intellectual content.
Conflicts of interestJMFG has received honoraria for speaking engagements and funding for conference attendance from Laboratories Esteve, Mundipharma, AstraZeneca, Boehringer Ingelheim, Ferrer, Menarini, Rovi, GSK, Chiesi, Novartis, and Gebro Pharma.
FJCG declares that he has received speaking and advisory fees and financial assistance to attend congresses from AstraZeneca, GSK, Novartis, FAES, Chiesi, Mundipharma, TEVA, Grifols, Boehringer Ingelheim and Gebro.
RG declares that he has received speaking and advisory fees and financial assistance to attend congresses from AstraZeneca, GSK, Novartis, FAES, Chiesi, Mundipharma, Menarini, TEVA, Grifols, Ferrer, Boehringer Ingelheim, Rovi and Gebro.
LMC declares that he has received speaking and advisory fees and financial assistance to attend congresses from FAES, Pari, Chiesi and Zambon.
MMO declares that she has received speaking and advisory fees and financial assistance to attend congresses from AstraZeneca, GSK, Chiesi and Menarini.
JMD declares that he has received grants, speaking and advisory fees, and assistance to attend congresses from Adamed, AstraZeneca, BIAL, Boehringer Ingelheim, Chiesi, FAES, Ferrer, Fresenius, Gebro, Grifols, GSK, Janssen, Menarini, Mundipharma, Novartis, Neuraxpharm, Sanofi, Roche, Rovi, Teva, Pfizer, and Zambon. JMD is a member of the editorial board of Open Respiratory Archives and declares that he has remained outside the evaluation and decision-making process in relation to this article.
GSLV declares that he has received speaking and advisory fees and financial assistance to attend congresses from GSK, Chiesi, FAES, Boehringer Ingelheim and BIAL.
JMTM declares that he has received speaking and advisory fees and financial assistance to attend congresses from AstraZeneca, GSK, FAES, Chiesi and BIAL.
AHF declares that he has received speaking and advisory fees and financial assistance to attend congresses from AstraZeneca, GSK, FAES, Chiesi, Boehringer Ingelheim, and Gebro.