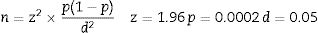Bell's palsy is the most common cause of peripheral facial palsy. Studies emphasis on the role of herpes simplex virus and herpes zoster in the pathogenesis of this disease. Gamma interferon plays an important role in determining the type of immune response against foreign invaders and intrinsic factors. Regarding increment of IFNγ level in acute viral diseases, we designed this study to assess the IFNγ levels and its relation to the intensity of neurodiagnostic findings in patients with Bell's palsy.
Methods30 patients in acute phase of Bell's palsy were selected and 5ml blood was obtained in the first 72h after diagnosis and right before the beginning of the treatment. Then Gamma interferon was measured and followed by nerve conduction study (NCS) 6 days after Bell's palsy onset.
ResultsThere was no significant relationship between gender of subjects and serum level of Gamma interferon with the intensity of entanglement in O. oculi and O. oris muscles. Also, there was no meaningful relationship between age and gender of patients and symptomatic factors with serum level of IFNγ.
ConclusionIt seems there is no significant relationship between immmunoserologic changes (serum level of IFNγ) and electrophysiological indices (NCS) in the acute stage of Bell's palsy.
La parálisis de Bell es la causa más común de parálisis facial periférica. Los estudios hacen hincapié en el papel del virus del herpes simple y del herpes zóster en la patogenia de esta enfermedad. El interferón gamma desempeña un papel importante en la determinación del tipo de respuesta inmune contra invasores extraños y factores intrínsecos. Con respecto al incremento del nivel de interferón gamma (IFNγ) en enfermedades virales agudas, diseñamos este estudio para evaluar los niveles de IFNγ y su relación con la intensidad de los hallazgos neurodiagnósticos en pacientes con parálisis de Bell.
MétodosSe seleccionaron 30 pacientes en fase aguda de parálisis de Bell y se obtuvieron 5 ml de sangre en las primeras 72 horas posteriores al diagnóstico, y justo antes del inicio del tratamiento. Luego se midió el IFNγ y se siguió con un estudio de conducción nerviosa (NCS) 6 días después de la aparición de la parálisis de Bell.
ResultadosNo hubo una relación significativa entre el sexo de los sujetos y el nivel sérico de IFNγ con la intensidad del entrelazamiento en los músculos O. oculi y O. oris. Además, no hubo una relación significativa entre la edad y el sexo de los pacientes y los factores sintomáticos con el nivel sérico de IFNγ.
ConclusiónParece que no existe una relación significativa entre los cambios inmunoserológicos (nivel sérico de IFNγ) y los índices electrofisiológicos (NCS) en la etapa aguda de la parálisis de Bell.
Bell's palsy (BP) is the most common cause of peripheral facial palsy, with the proportion of 62–93%.1 The annual incidence of Bell's palsy is 14–25 cases per hundred thousand individuals in the population.2 Its incidence slightly increases with age and there is a little difference between two sexes.3 Also, the incidence has some increments in the winter.4 Bell's palsy in 80–90% of cases is acute and unilateral. Etiology and treatment of Bell's palsy are controversial. Some of Bell's palsy causes include inflammation, autoimmunity, viral infections and ischemia.5
All patients have some degrees of nerve fiber degeneration pathologically; however, the considered reasons for this pathologic situation were not in unanimous agreement. For example, suspicion to the viral agent has been proposed a long time ago but the existence of this mechanism has been established certainly only in the last few years. In the Burgess et al. study, according to Ropper et al. Herpes simplex virus (HSV) DNA has been found in a patient with Bell's palsy. Also, according to Ropper et al., Murakami et al. produced facial paralysis by inoculating HSV into the ears and tongues of mice which lead to founding the virus antigens in facial nerve and geniculate ganglia.6
The theory of viral infection is more acceptable than others. In fact, there are controversies about the cause of the paralysis which is the result of viral infection alone or ischemic neuropathy secondary to viral infection.7
Studies emphasis on the role of HSV and Herpes Zoster virus (VZV) in this disease. Serological studies indicate that a high percentage of people with Bell's palsy have antibodies for HSV (compared to control group).8 Because there is no clear evidence about increasing the amount of specific antibody in Bell's palsy, the disease can be the result of the delayed activity of the virus. According to Aviel et al.9 Adour et al.10 and Vahlne et al.5 assumed that BP is probably caused by reactivation of a latent herpes simplex virus without increased antibody titers.11 This theory is strengthened by increasing serum interferon at the time of Bell's palsy.12,13
Studies show that Bell's palsy may have an autoimmune basis. Recent evidence has reported that amount of serum complements have increased in Bell's palsy. During the first 24h of Bell's palsy, numbers of T lymphocytes reduce and B lymphocytes of the peripheral blood increase. But there was no difference between the number of lymphocytes in the patients and also the relationship between this percentage and patient recovery time has not been established.13
IL-1, IL-6, IL-8, TNFα, and IFNγ serum levels increase in patients with Bell's palsy.14 The cell-mediated immune response to Bell's palsy has been reported in studies.15,16 Also, there is evidence of changing in levels of some subtypes of lymphocytes in the acute phase of Bell's palsy.11 Reduction in the amount of T (CD3) and T Helper (CD4) cells in these patients compared to healthy subjects has been proven and it indicates the role of the immune response in Bell's palsy.17 So that some of the studies introduce Bell's palsy as a cell-mediated autoimmune neuritis and show its obvious role in cell-mediated immunity.9
Gamma interferon (IFNγ) is an important cytokine that plays an important role in determining the type of immune response against foreign invaders and intrinsic factors and is secreted by lymphocytes Th1, CD8+ Tc cells and NK cells. Many studies on the effects of various factors such as viruses, bacteria, drugs, antigens, superantigenic genes, mitogen, allergens, and cancer have been carried out about the expression of Gamma interferon gene, in situations of in vitro and in vivo. Studies revealed that secretion of Gamma interferon increased by activated T cells in different models of the virus.18 Recently it was shown that T cells secrete more Gamma interferon in HSV1 infection and it deactivates the HSV1 virus in the nerve ganglia.19,20 Gamma interferon plays an important role in T helper 1 cells’ development.21 So that in response to intracellular microbes, IL12 is secreted by dendritic cells. Macrophages and NK cells also secrete INF-gamma. These cytokines stimulate primary T cells to differentiate into T helper 1 cells. Gamma interferon which is made by T helper 1 cells reinforces this response and prevents the evolution of T helper 2 cells. Also, it enhances phagocytosis in macrophages and stimulates B cells’ antibodies production.22 IFNγ not only plays an important role in the acute phase of viral infections such as herpes viral infection but also plays an important role in the latent phase of viruses such as HSV, as determined by reverse transcriptase PCR, IFN-g and tumor necrosis factor alpha transcripts were present in trigeminal ganglia during both acute and latent HSV-1 infection.23
Measuring performance of facial nerve in Bell's palsy, using electrical tests like Electroneuronography (ENoG) will be accompanied by various errors.24 The difficulty of judgment about two-sided entanglements, difficulty in inducing electrical stimulation at two points because of a high degree of nerve anastomosis in the parotid gland and short time period between excitations, and complexity of the arrangement of facial muscles are some examples. It seems that measuring CMAP amplitude is a good way for assessment of the seventh pair of cranial nerves in Bell's palsy.19,20
Therefore, the aim of this study was to assess the IFNγ level and its relation with severity of NCS abnormalities in patients with Bell's palsy.
MethodsStudy designIn this cross-sectional study carried out after getting ethnical approve of the ethics committee of the Zanjan University of Medical Science. Written consent of patients for participating in the investigation was also obtained.
SettingThe study was implemented at Vali-e-Asr university Hospital in Zanjan, Iran. General and demographic information was gathered and 5ml blood was obtained from patients in the first 72h after diagnosis and right before treatment. Blood samples were centrifuged and serum was stored at −80°C for Gamma interferon level measurement. We implemented nerve conduction study (NCS) 6 days after Bell's palsy diagnosis.
Participants30 patients with Bell's palsy in acute phase)who referred to the hospital in the first 24h after the onset of facial paralysis) selected consecutively of whom had referred to the emergency, neurology and ENT clinics and emergency service of Vali-e-Asr Hospital in Zanjan city. Inclusion criteria were proved idiopathic paralysis (Bell's palsy) with the exclusion of other causes of 7th cranial nerve palsy such as direct trauma, otitis media, brain stem lesions and malignant lesions. Patients with systemic infections were excluded as well. All possible causes were ruled out by history taking, systemic and complete neurologic examinations and neuroimaging if necessary.
VariablesWe carried out all nerve conduction studies (NCS) by only one NCS/EMG equipment and the same neurologist at Zanjan Vali-e Asr University Hospital, 6 days after onset of Bell's palsy in which the ratio of CMAP amplitude of Orbicularis Oris or Orbicularis Oculi muscles in the affected side to unaffected one was measured (under supramaximal stimulation). The scale of the assessment was CMAP amplitude. Less measured amplitude ratio of affected side to healthy was the indicator of less intensity involvement. These NCS results were categorized into 3 groups: poor prognosis (<10%), moderate prognosis (10–50%) and good prognosis (>50%).
We used Boster Immunoleader EK0373 Elisa kit for assessing the Gamma interferon serum level. Normal range according to instruction was 15.6–1000pg/ml and accuracy was 2pg/ml.
BiasThere were some biases including lack of enough accessible patients, no measuring EMG and Blink Reflex and lack of high accuracy in timing for doing next examinations after start point. Also, we were not able to follow up our patients after 3–6 months after initiation of the study.
Study sizeFor the low prevalence of Bell's palsy, we participated all patients who referred to neurology and ENT clinics and emergency service of Vali-e-Asr hospital, Zanjan in the years 2014–2015 with mentioned criteria. Selection method was consecutive and simple non-randomized sampling.
Here is the estimated sample size which is not rational for study so we decided to select participants according to the aforementioned method:
Calculated study size was 0.35.
Statistical methodsInformation was analyzed by using frequency tables, Index of dispersion and Central Tendency and odds Ratio and CI 5% were calculated by SPSS 16 software. For data analysis, linear regression test, One Way, and Chi-square were used.
ResultsAll participants had proved idiopathic paralysis (Bell's palsy) in whom other facial nerve palsy's causes such as direct trauma, otitis media, brainstem lesions, and neoplastic lesions of nerve. 7 were ruled out by history taking, systemic, and 12 cranial nerves examinations.
Among 30 patients participating in this study 19 were men versus 11 women with mean age of 45±17 years (the youngest was 20 years old and the oldest one 80). Having the history of atherosclerotic diseases 9 patients (30%) had a positive documented history of these diseases like diabetes mellitus, hypertension, hyperlipidemia, and stroke or ischemic heart diseases. About the family history of Bell's palsy, only 1 patient (3.3%) had a positive family history. Facial nerve palsy was seen in 13 patients on the right side (43.3%) and in 17 patients on the left side (56.7%). Frequency distribution of accompanying symptoms such as diarrhea or flu-like syndrome during the last week before disease, postauricular pain, taste disorders, Hyperacusis, and hyperlacrimation is shown in Table 1.
Frequency distribution of accompanying symptoms.
| Clinical sign | +/− | N (%) |
|---|---|---|
| Diarrhea or flu-like syndrome during the last week before disease | + | (3.3)1 |
| − | (96.7)29 | |
| Postauricular pain | + | (60)18 |
| − | (40)12 | |
| Taste disorders | + | (36.7)11 |
| − | (63.3)19 | |
| Hyperacusis | + | (16.7)5 |
| − | (83.3)25 | |
| Hyperlacrimation | + | (60)18 |
| − | (40)12 | |
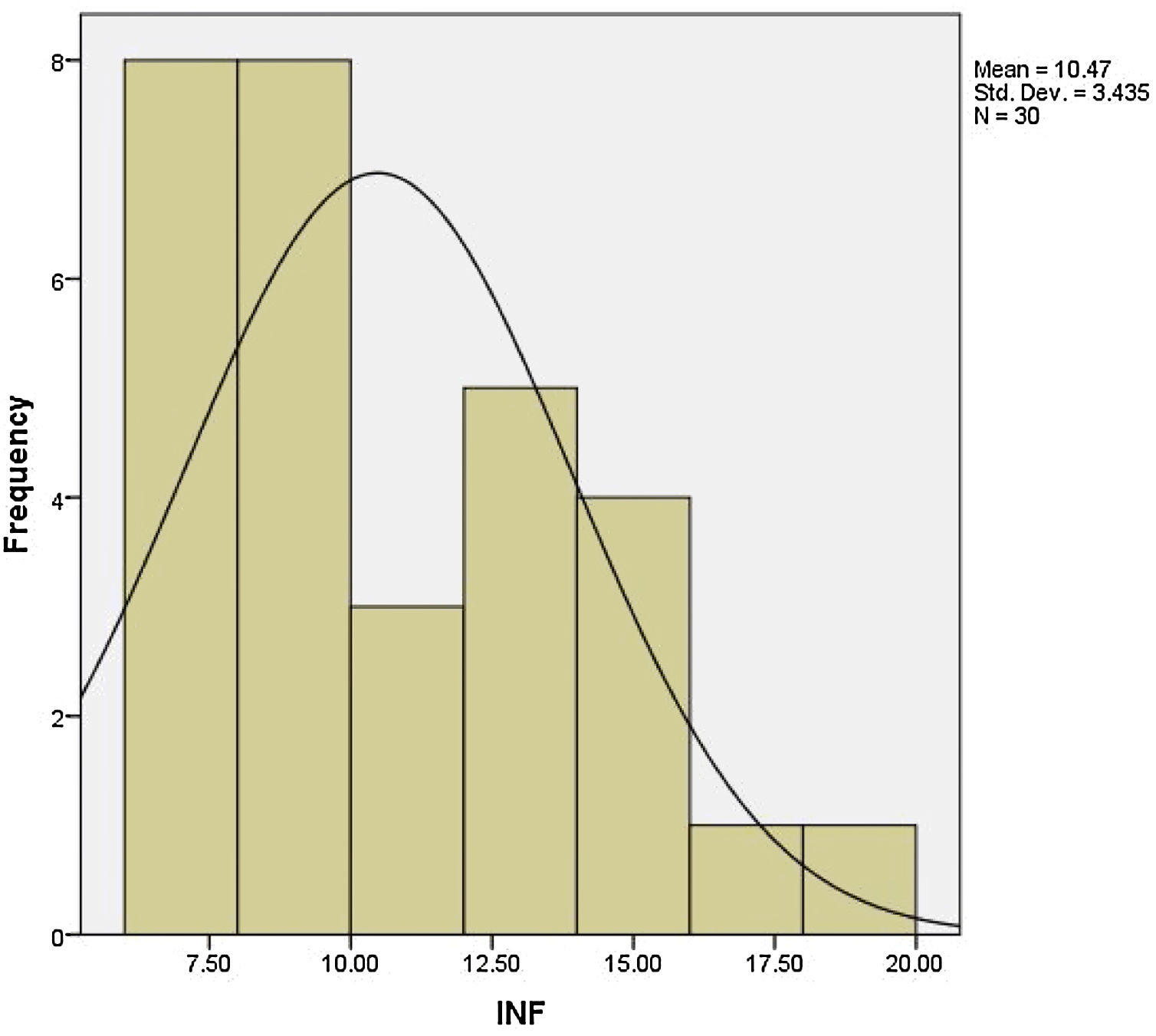
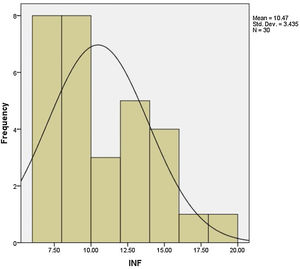
We performed NCS on Orbicularis Oris and Orbicularis Oculi muscles for all subjects enrolled in this study on day 6 of disease course and calculate the proportion of the amplitude of the compound muscle action potential (CMAP) of the affected side to the healthy side. Patients were divided into 3 groups of good prognosis (ratio rate of >50%), moderate prognosis (ratio rate of 10–50%), and poor prognosis (ratio rate of <10%) based on the severity of involvement. This ratio is also calculated in both muscles and also based on the highest intensity of entanglement totally. Serum level of Gamma interferon in the first 72h of the disease was measured in all patients with ELISA method and the least level of that was 6.5 and the highest level was 19.2 with a mean level of Gamma interferon 10.47±9.43. Frequency distribution of levels of Gamma interferon in all patients is shown in Fig. 1.
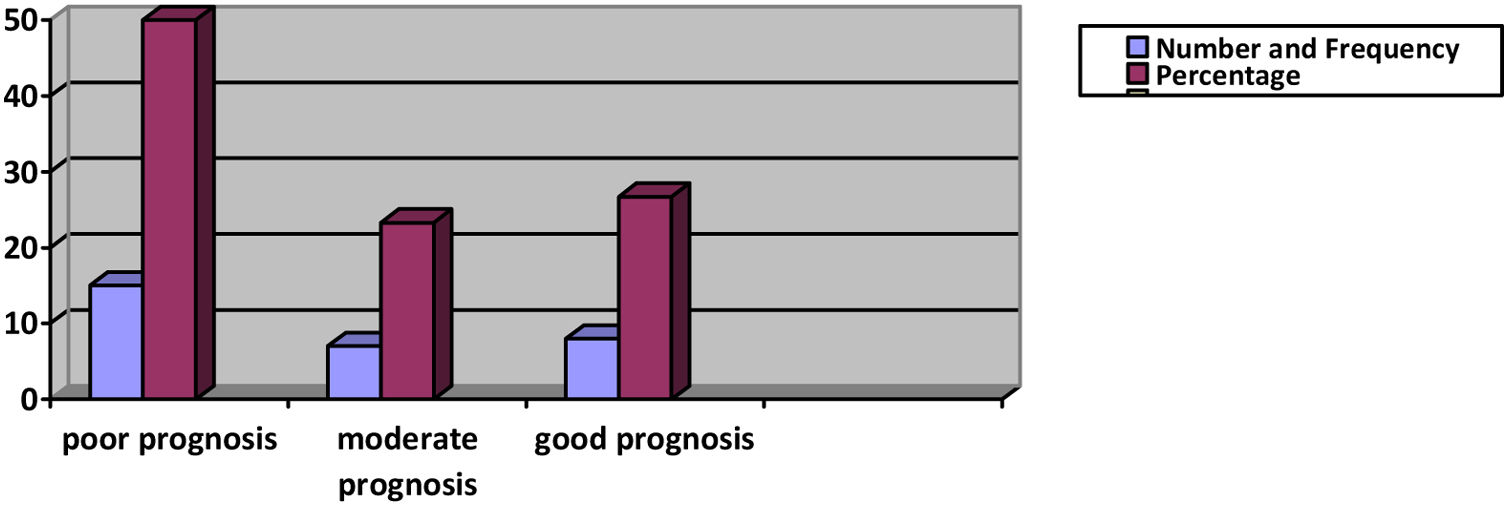
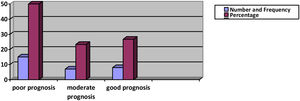
Based on the amplitude ratio of the affected to the unaffected side for O. oculi muscle CMAPs, the highest rate of entanglement was 0.03 and the least rate was 0.92, respectively. In this study 9 patients situated in poor, 9 in moderate and 12 in good prognostic categories. In O. oris muscle the least ratio was 0.04 (worst damage) and the highest ratio was 0.97 (minimal damage). According to the NCS results in 9 patients categorized in poor prognosis group, 9 in moderate and 12 in good prognosis group. We also classified patients based on the most intensity of injury in either the O. oris or O. oculi muscles. Based on this classification, 15 patients were in poor, 7 in moderate, and 8 in good prognosis groups (Fig. 2).
Studying the relationship between the intensity of involvement in O. oculi muscle (based on the NCS results) and gender of patients, there were 4 males vs 5 females in poor prognosis group, 7 men vs 2 women in moderate prognosis group, and 8 male vs 4 female in good prognosis group. Using chi-square test there was no significant relationship between gender and severity of injury in O. oculi muscle (p=0.325). We also evaluated this relationship for O. oris muscle and as well as for the highest rate of involvement (based on the NCS) in regards to gender; However, there was not any significant relationship. We also studied the relationship between age, affected side, family history, past medical history, postauricular pain, flu-like symptoms or diarrhea, taste disorders, Hyperacusis, and hyperlacrimation with intensity of entanglement of O. oculi and O. oris muscles and the highest rate of entanglement (based on the NCS results) and found that none of these factors had significant relation with intensity of entanglement.
Studying the relationship between serum levels of IFN γ with age based on the analysis with linear regression test indicated that there was no meaningful relationship between them (p-value=0.426). About the relationship between gender of patients and serum level of IFN γ, the mean serum level of Gamma interferon in men was 10.52±4.01 with the least level of 6.5 and the highest level of 19.2. The mean serum level of Gamma interferon in women group was 10.38±2.29 with the least level of 7 and the highest level was 13.6. Finally, the analysis with ONE-WAY test did not show any significant relationship between serum levels of IFN γ and gender (p-value=0.914). The results of studying the relationship between serum level of IFN γ and symptomatic factors of the subjects have been presented in Table 2 which declares no meaningful relationship in none of the accomplished analysis.
The relationship between serum level of INF-γ and symptomatic history.
| Variables | N | Mean of IFN | Standard deviation | Min | Max | p value |
|---|---|---|---|---|---|---|
| Familial history | ||||||
| + | 1 | – | – | 7 | 7 | 0.914 |
| − | 29 | 10.59 | 3.43 | 6.50 | 19.20 | |
| Past medical history | ||||||
| + | 9 | 11.13 | 4.19 | 7.00 | 19.02 | 0.500 |
| − | 21 | 10.19 | 3.12 | 6.50 | 16.30 | |
| Flue or diarrhea | ||||||
| + | 1 | – | – | 0.948 | ||
| − | 29 | 10.46 | 3.49 | 6.50 | 19.20 | |
| Postauricular pain | ||||||
| + | 18 | 10.45 | 3.58 | 6.50 | 19.20 | 0.965 |
| − | 12 | 10.50 | 3.34 | 7.00 | 16.30 | |
| Taste disorders | ||||||
| + | 11 | 9.11 | 2.23 | 7 | 13.1 | 0.101 |
| − | 19 | 11.25 | 3.80 | 6.50 | 19.20 | |
| Hyperacusis | ||||||
| + | 5 | 9.66 | 2.86 | 7 | 13.10 | 0.571 |
| − | 25 | 10.63 | 3.56 | 6.50 | 19.20 | |
| Hyperlacrimation | ||||||
| + | 18 | 10.88 | 3.03 | 7 | 15.6 | 0.433 |
| − | 12 | 9.85 | 4.02 | 6.5 | 19.20 | |
Key study about the relationship between the serum level of IFNγ and NCS results was done in some domains. First, the relationship between serum levels of Gamma interferon with NCS results in O. oculi and O. oris muscles and the minimum amplitude ratio between each of them was evaluated in each patient. Then the relationship between serum level of IFN γ and severity of damage according to the three classes of prognosis in each of the O. oculi and O. oris muscles and the highest final severity were analyzed (Table 3).
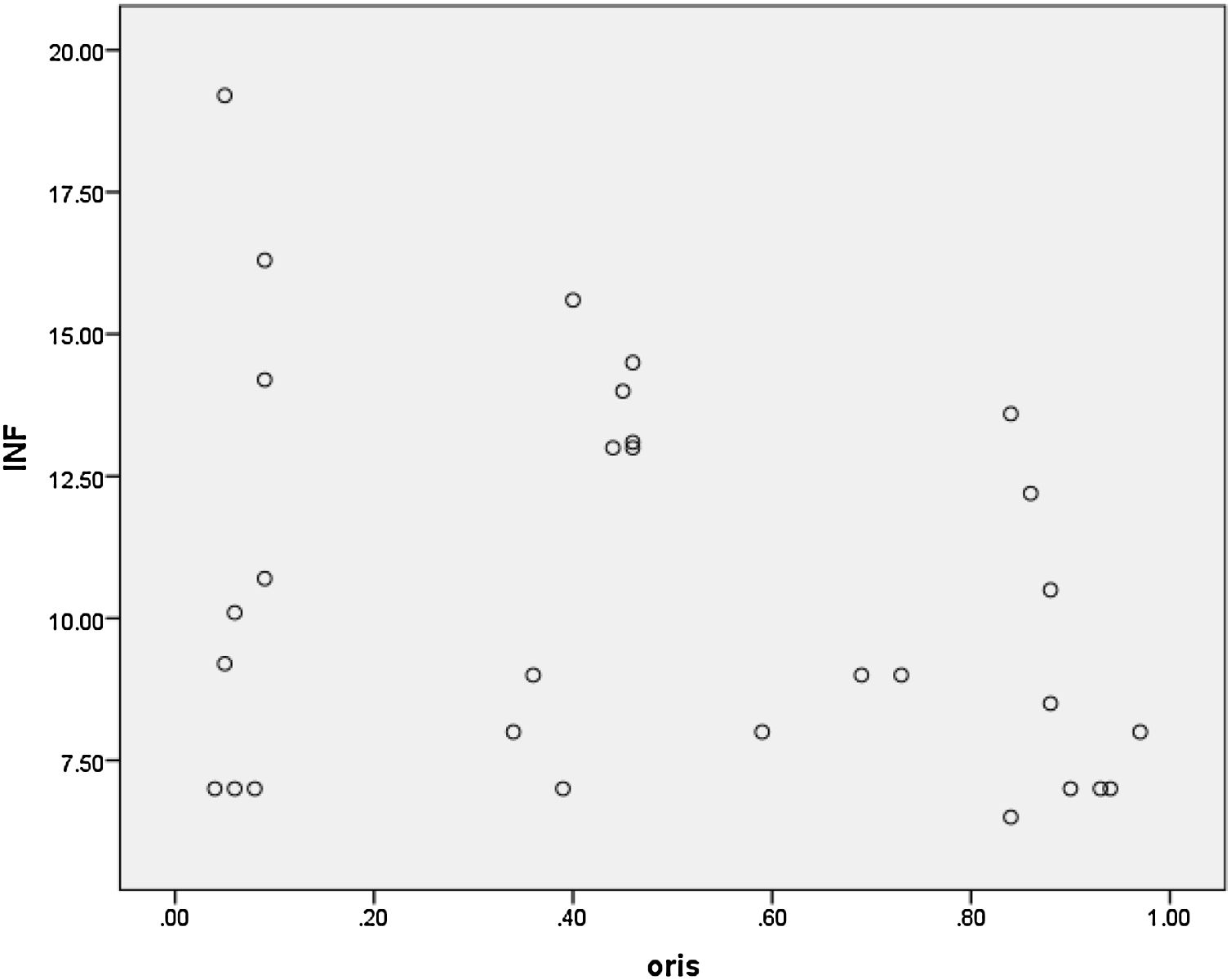
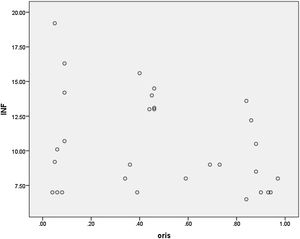
Using linear regression test, there was no relationship between serum levels of IFN γ and NCS results in percentage in O. oculi muscle and in all patients (p-value=0.387). Studying this relationship in O. oris muscle and in all patients based on the coefficient of correlation (R2=0.084) disclosed 8% change of IFN γ is justified with NCS results but there was no relationship via using linear regression test (p-value=0.06). Fig. 3 shows the correlation between results of NCS and serum levels of Gamma interferon which indicates few relationships between them.
In linear regression test, the least ratio of CMAP amplitude (highest rate of involvement)in each of the O. oris and O. oculi muscles did not affirm any relationship with serum level of INF-γ (p-value=0.205). According to the NCS results in O. oculi muscle, mean serum level of IFN γ in poor prognosis group was 9.34±2.73 with a minimum of 7 and maximum of 14.5. In moderate prognosis group, mean serum levels of Gamma interferon was 12.08±4.32 with a minimum of 7 and maximum of 19.2. These numbers were 10.1±3.06, 6.5 and 16.3 in good prognosis group, respectively. Finally, the one-way analysis did not show any meaningful relationship between the intensity of involvement of O. oculi muscle and serum level of IFN γ (p-value=0.218).
Studying the relationship between serum level of IFN γ and maximum severity of damage based on the NCS results in both muscles, mean serum level of Gamma interferon in poor prognosis group was 10.71±3.58 with a minimum of 7 and maximum of 19.2. In moderate prognosis group, mean serum level of IFN γ was 11.38±3.23 (minimum=7; maximum=15.6) and in the good prognosis group, mean serum level of IFN γ was 9.2±2.63 (minimum=5.6; maximum=13.6). Final analysis via one-way test implied no meaningful relationship between these two variables (p-value=0.459).
DiscussionThis study was a cross-sectional study which aimed to determine serum levels of Gamma interferon in the acute phase of Bell's palsy and evaluating its proportionality with the severity of nerve conduction study disturbances.
In this study, 19 men and 11 women with a mean age of 45 years were enrolled and the serum level of interferon-gamma was measured by ELISA, within 72h following the onset of illness.
Jonsson and colleagues in a study in 1989 evaluated serum levels of gamma interferon in patients with Bell's palsy. In the study, levels of IFNγ was evaluated in 91 patients with Bell's palsy. It was shown that level of IFNγ in acute and convalescent phase was significantly increased in patients compared to controls.16 Their study advantages were larger study size and comparing with the control group, differences in results of two studies are justifiable by these.
In another study by Aviel and colleagues in 1989, they evaluated the serum level of IFN-γ and anti-viral activity in 32 patients with Bell's palsy, after 72h from the beginning of the disease. Serum level of Gamma interferon or antiviral activity increased in 23 patients (72%). In this study, normal level of IFN-γ was 16 and the average was 95 which began to decrease 72h after onset of disease.17 Our study has some similar features, such as study size and the time of IFN-γ measurement. But we have not reported our results quantitatively unlike this study.
In other studies, such as Greco and colleagues in 2012, they implied the role of humoral and cellular immunity and Gamma interferon in Bell's palsy.25
Our study was consistent with the above study in IFN-γ increment in patients with Bell's palsy. We considered the first 72h of an appropriate time interval for evaluating INF-γ levels. Thus, evaluating the meaningfulness of serum level of INF-γ increment was not possible because there was no control group in our study.
We performed nerve conduction study (NCS) 6 days after Bell's palsy initiation in which the ratio of CMAP amplitude of Orbicularis Oris or Orbicularis Oculi muscles of affected side to normal was measured (in supramaximal stimulation). In previous studies, NCS was used to assess the severity of Bell's palsy or as a prognostic factor of the disease. Ceccanti and colleagues in 2013 studied the predictive role of neurophysiology in Bell's palsy disease. 92 patients were recruited and NCS was carried out for Orbicularis oris and Orbicularis oculi muscles. They concluded that amplitude reduction in the affected side compared to the healthy side was related to the outcome of patients with Bell's palsy.26 Joachims and colleagues in a study in 2009 on 100 patients with Bell's palsy, found that NCS had a significant role in early diagnosis and determining the severity of the disease. Patients with more problems in NCS showing less improvement during the study period.27 In our research, NCS results were used to evaluate severity and prognosis of patients and categorize them. But this measurement did not turn to the basis for measuring treatment response and recovery and it was not re-evaluated which is the limitation of our study.
In the current study, the intensity of nerve damage had no correlation with variables including age, gender, affected side, family history, history of underlying disease, symptoms, abnormal taste, Hyperacusis, and tearing. Sang et al. in 2015 studied the prognostic value of electrophysiology of Bell's palsy in O. oculi muscle and nasolabial fold. They reviewed the electroneurographic results of 81 patients at the beginning and end of the study and did not found any significant relationship between electroneugraphic results and age, sex, affected side, and time.28 This study confirms our findings on the lack of relation between NCS results and variables.
In 2014 Fujiwara and colleagues conducted a study about prognostic factors of Bell's palsy disease in 679 patients. Effects of age, gender, affected side, past medical history, and severity of the disease on the prognosis of patients were assessed. The severity of the disease was relevant to non-healing condition through one week. Age, sex, underlying disease, affected side, and underlying diseases had no relevance with the outcome.29 The results of our study also showed the lack of relationship between the intensity of the disease and mentioned variables. In another study, Jafari and colleagues in 2004 evaluated the role of electrodiagnosis and taste disturbance in the prognosis and diagnosis of Bell's palsy. The study was conducted on 44 patients with Bell's palsy and amplitude reduction on the affected side was the basis of the study. Patients underwent electrical stimulation and EMG at the beginning and three weeks after the onset of Bell's palsy. In this study, significant associations were found between following: (1) age and the normal EMG response; (2) reduction and electromyography after 3 weeks; (3) impaired sense of taste and improvement of symptoms and severity of disease after 3 weeks.30 The results of this study are different from our findings. Differences could be due to: (1) patients in our study did not undergo follow-up and re-test after 3 weeks; (2) differences in the aspects of follow-up and evaluation of the relationship between the severity of the disorder with taste disturbances and age at the beginning of the study.
No similar study has been done in regards to the relationship between interferon-gamma and degree of disturbances in electrophysiologic tests. In our study, the relation between serum levels of IFN-γ and all variables were evaluated. The relationship was evaluated in two muscles, O. oris and O. oculi.
In spite of no significant relevance, the noteworthy point was the weak relation between NCS results in O. oris muscle and serum IFN-γ levels. According to p-value=0.06 and R2=0.084, 8% changes in gamma interferon level can be described by the NCS results of O. oris muscle. This correlation coefficient shows that these two variables were not associated with the sample size. So it cannot be expected that making the number of samples larger can make significant the relevance. But with the methodological modification, for example by using a control group or follow-up and variables’ re-evaluation after 3 weeks, significant results can be expected. Also in this study, most decreased amplitudes were selected among the results of NCS for O. oculi and O. oris muscles and its relevance with serum level of interferon-gamma was evaluated, in which significant relevance was not detected. Due to the lack of significant relationship in two muscles, it can be deduced from these 3 assessments that serum level of IFN-γ would not be relevant to the intensity of nerves disturbance even with changing sample size (according to correlation coefficient) and examined muscle.
In this study, the relationship between serum level of IFN-γ with variables, i.e. age, sex, affected side, family history, history of underlying disease, symptoms, abnormal taste, Hyperacusis, and tearing was examined. Similar to the aforementioned studies which showed no relationship between the intensity of disease and mentioned variables, it was expectable that serum level of IFN-γ had no relation with variables. Although it cannot be ignored that age has an effect on serum level of Gamma Interferon.
In this study, we categorized patients according to NCS results in O. oculi and O. oris muscles and the most decreased amplitude into three prognostic groups: poor, medium, and good; then, relevance with serum level of Gamma interferon was assessed in each group, separately. Based on the current study, there was no significant relationship between variables. Though, regarding high serum level of IFN-γ in patients with Bell's palsy and the relation between involvement severity and NCS results, we expected that there would be an association between these two variables. Considering that serum IFN-γ level is not high in all patients just in few, there is a possibility that low serum level of IFN-γ in some cases is a result of dissociation between these two variables. In other words, it is presumed that if patients with higher than normal serum levels of IFN-γ were enrolled, there would be relations between the serum level of interferon-gamma and involvement severity based on the NCS results.
Our study limitation was being impossible to re-evaluate patients for a long time due to cultural beliefs.
ConclusionsSerum level of IFN-γ has not any relation with severity and prognosis of Bell's palsy. Hence it cannot be used as therapeutic and surgical indications and it is not appropriate for early diagnosis of Bell's palsy and assessing differential diagnosis.
The following suggestions are offered for the design of future studies: (1) measurement of serum interferon and assessing NCS at the beginning of the disease, and after 3–4 weeks, (2) comparative study between patients and controls, (3) assessment of high Gamma interferon relation with nerve conduction disorders, (4) measuring the concentration of interferon gamma in tear and saliva at 1, 3, 7, 14 and 21 days following diagnosis, (5) enrolling narrower age spectrum, since immune response and etiology are different in younger patients, (6) measurement of IFN-γ in constant times because the immunologic cascades of cytokines are different in accordance to trigger and the paralysis etiology.
Authors’ contribution1. M.M. conceived the idea of this commentary, designed and performed data collection and statistical analysis & interpretation of the data & editing of manuscript; Manuscript writing and final approval of manuscript.
2. S.H. conceived, designed and did statistical analysis & data collection and drafted the manuscript.
3. N.A.S. contributed to statistical analysis & data collection and manuscript writing.
4. A.E. conceived, and executed data collection& interpretation of the data, editing of manuscript, manuscript writing, review and final approval of manuscript.
The corresponding author is a recipient of a research scholarship.
All authors agree to be accountable for all aspects of the work.
Grant support & financial disclosuresThis work was supported by Grant from the deputy of research and technology of Zanjan University of Medical Sciences.
Declaration of financial/other relationshipsAll authors have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
Conflict of interestsThe authors declare no conflict of interests.
We would like to appreciate all staffs of neurology and ENT clinics of Vali-e-Asr hospital and all patients and their families for their cooperation and sufferance.