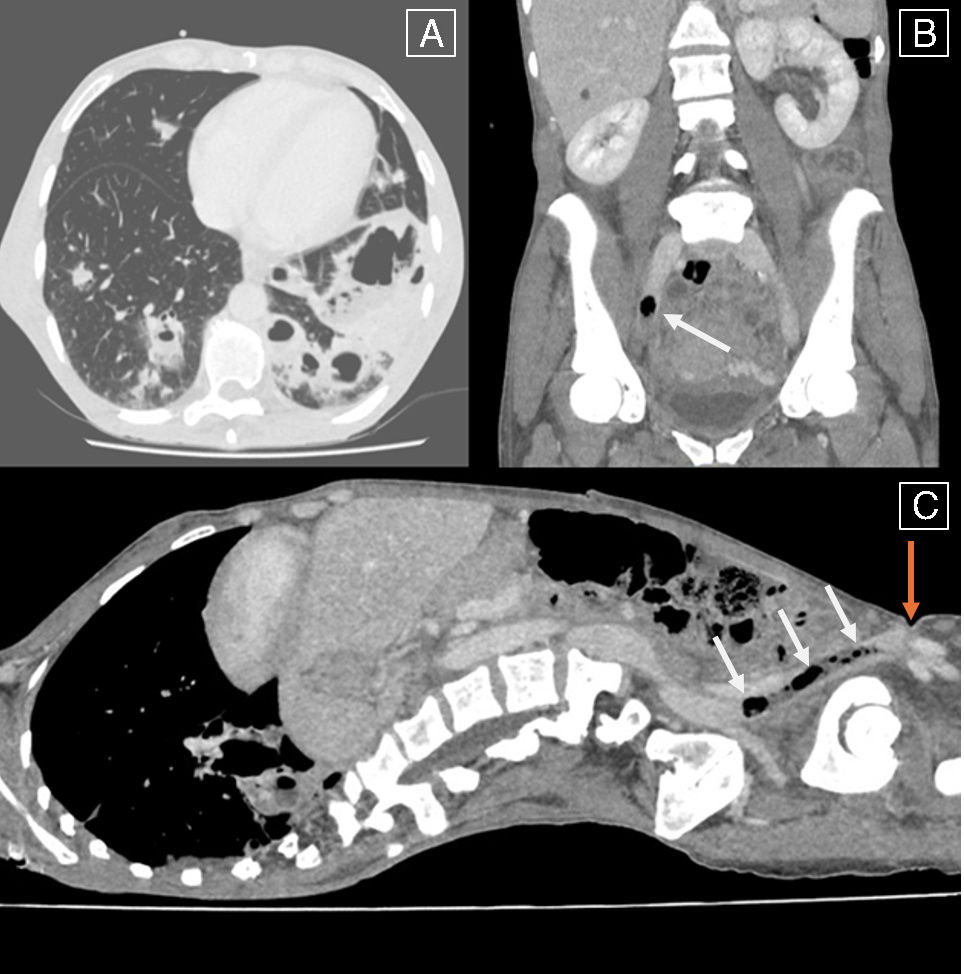
A 47-year-old woman, who is an intravenous drug user, presented to the emergency department with a 2-day history of fever and cough. Laboratory tests revealed a C-reactive protein level of 231mg/L (normal value <5mg/L). The chest scan revealed multiple pulmonary abscesses (Fig. 1, panel A). Blood cultures were positive for Streptococcus mitis, Granulicatella adiacens, and Lancefieldella parvula. Piperacillin–tazobactam antibiotherapy was initiated. A transesophageal echocardiogram ruled out endocarditis. The thoracoabdominal pelvic scan revealed (Fig. 1, panels B and C) multiple new pulmonary abscesses, septic pulmonary emboli, and a septic thrombosis with gas formation within the external iliac and common femoral veins (Fig. 1, panels B and C white arrows), secondary to drug injection in the groin area (Fig. 1, panels B and C orange arrow). Intravenous drug use is found in 25% of septic pulmonary embolism case while septic thrombophlebitis is found in 6% of cases. Curative anticoagulant therapy was initiated due to the size of the thrombus, even if the benefit in septic thrombophlebitis is still debated. However, hemoptysis occurred leading to the anticoagulant therapy discontinuation and the patient's condition improved rapidly. Complications, including hemoptysis, are frequent in septic pulmonary embolism with a 20% mortality rate. The antibiotherapy was switched to amoxicillin–clavulanic acid for 4 weeks. Unfortunately, the patient was lost to follow-up.
Ethical approvalNon opposition of the patient was obtained.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestVG declares personal fees from Pfizer outside the submitted word.