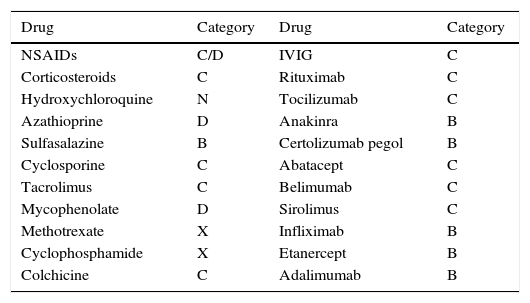
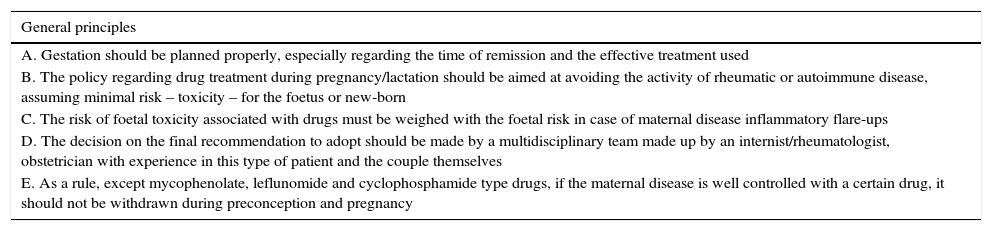
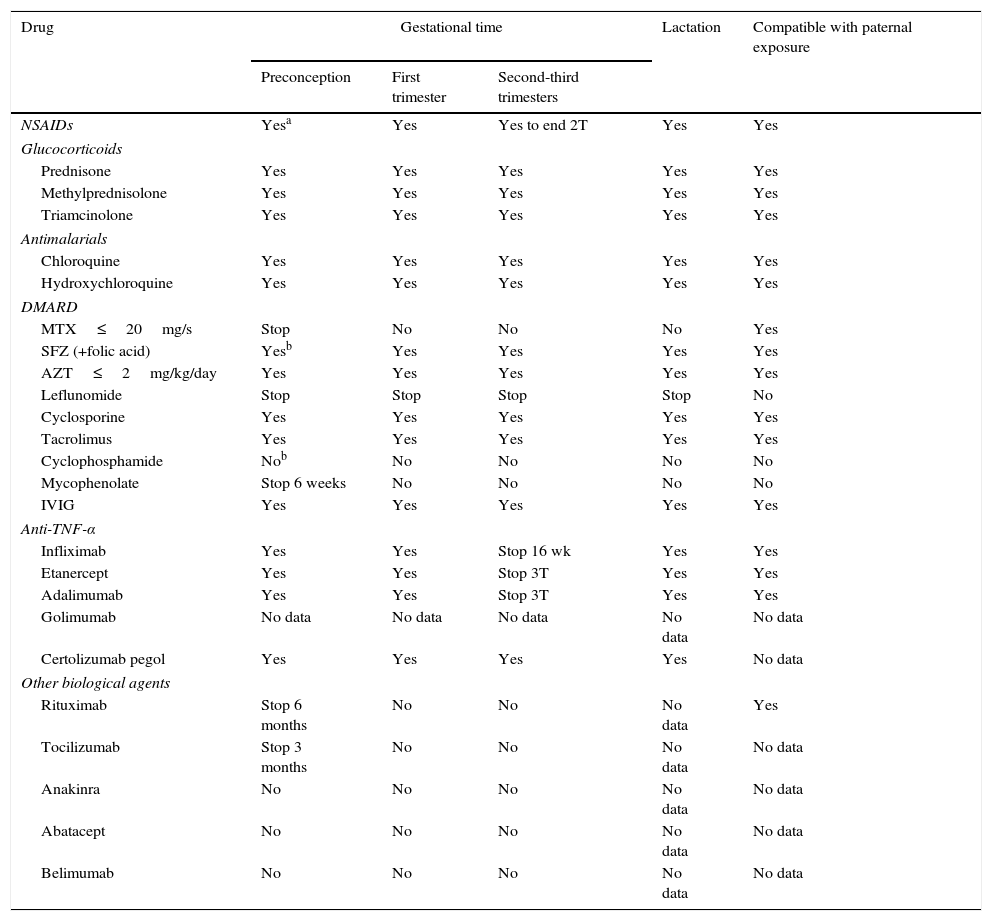
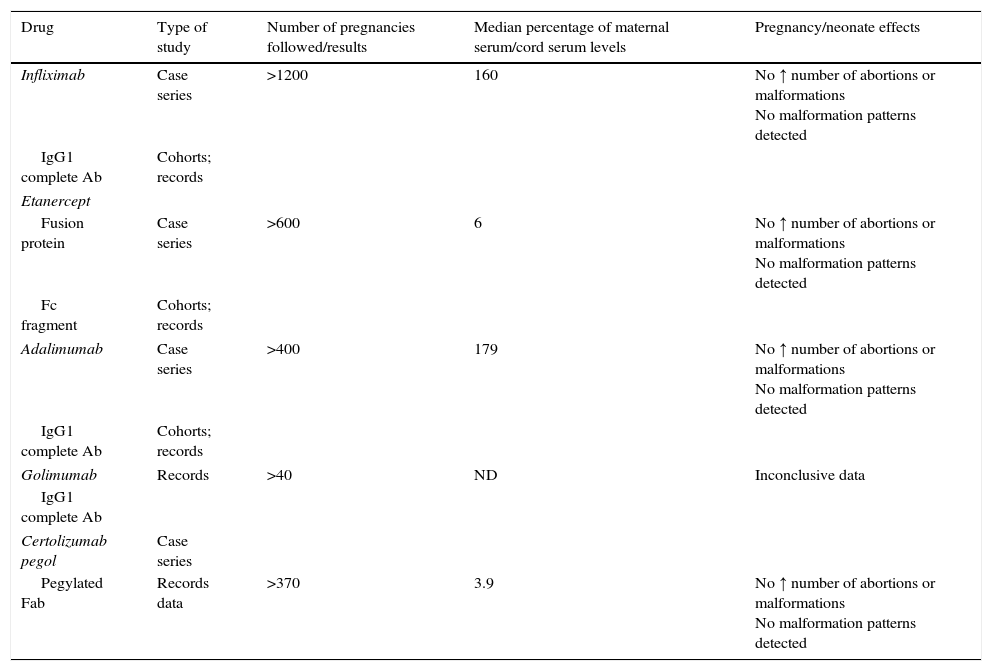
Rheumatic and systemic autoimmune diseases occur in women and, to a lesser degree, men of reproductive age. These disorders have to be clinically nonactive before conception, which is usually only possible after anti-inflammatory and immunosuppressive treatment. We must be alert since 50% of pregnancies are unplanned. Physicians should know the embryo-foetal toxicity of these drugs during pregnancy and lactation. This January 2016-updated review allows us to conclude that the majority of immunosuppressives available – anti-TNF inhibitors included – can be used before and during pregnancy, with the exception of cyclophosphamide, methotrexate, mycophenolate and leflunomide. Lactation is permitted with all drugs except methotrexate, leflunomide, mycophenolate and cyclophosphamide. Although data on abatacept, belimumab, rituximab, tocilizumab and anakinra are scant, preliminary reports agree on their safety during pregnancy and, probably, lactation. Cyclophosphamide and sulfasalazine apart, no negative effects on sperm quality, or embryo-foetal anomalies in men treated with immunosuppressives have been described.
Las enfermedades reumáticas y autoinmunitarias afectan a mujeres y, en menor medida, también a hombres en edad fértil. Estas deben estar sin actividad clínica antes de la concepción, lo que suele conseguirse con fármacos inmunodepresores/antiinflamatorios. Un 50% de los embarazos en este colectivo no serán planificados. Los profesionales debemos conocer los efectos embrio/fetotóxicos de estos fármacos, así como sus consecuencias negativas sobre la gestación y la lactancia. Esta revisión actualizada hasta enero de 2016 permite concluir que la mayoría de los inmunodepresores disponibles, incluidos los inhibidores del TNF, se pueden utilizar antes y durante la gestación, con la excepción de ciclofosfamida, metotrexato, micofenolato y leflunomida. Se puede lactar exceptuando si se usa metotrexato, leflunomida, micofenolato y ciclofosfamida. Parece que abatacept, belimumab, rituximab, tocilizumab y anakinra son seguros durante la gestación y la lactancia. Exceptuando ciclofosfamida y sulfasalazina, no se han comunicado efectos sobre la calidad espermática ni un aumento de embriofetopatías en hombres tratados con inmunodepresores.