Tranexamic acid (TXA) is commonly used to control postoperative blood loss in total knee arthroplasty. In order to avoid adverse effects associated with intravenous administration, topical use has been proposed as an alternative. Our aim was to evaluate the efficacy and safety of topical TXA in total knee arthroplasty.
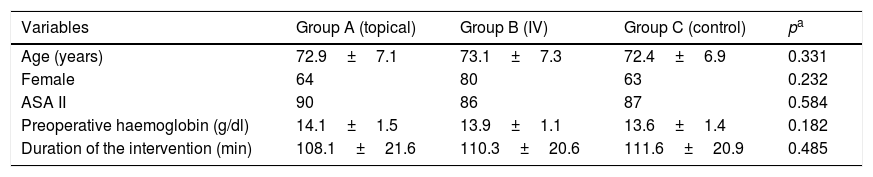
Material and methodsA total of 90 patients scheduled for unilateral total knee arthroplasty were included in a prospective randomized study. All surgeries were performed under spinal anaesthesia, tourniquet and the same postoperative protocol. Patients were allocated to one of the 3 groups according to the application of TXA: group A (n=30) 1g of topical TXA; group B (n=30) 1g of TXA intravenous and in group C or the control group (n=30) no drug was administrated. Parameters related to blood loss and drain outputs were compared between the 3 groups.
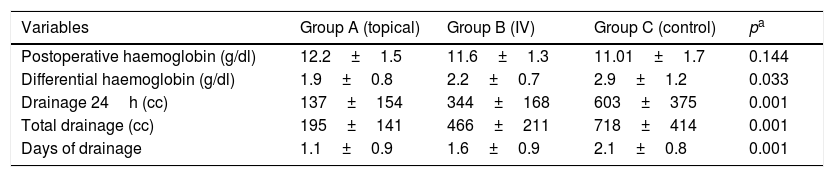
ResultsThe results revealed that post-operative decrease in haemoglobin level was significantly lower in group A (1.95g/dL) than group B (2.25g/dL) and group C (2.96g/dL), p<0.01. Total postoperative blood loss was lower in group A (195mL) than group B (466mL) and group C (718mL), p<0.01. There was no significant difference in complications and allogenic blood transfusion rate between the 3 groups.
ConclusionsAccording to the results, topical application of 1g TXA significantly reduced blood loss in patients undergoing total knee arthroplasty more than intravenous or no administration of TXA.
La administración de ácido tranexámico (ATX) es efectiva reduciendo la pérdida de sangre en la artroplastia de rodilla. Con el fin de evitar los efectos adversos de la administración intravenosa, se ha propuesto el uso tópico del mismo. Nuestro objetivo es evaluar la eficacia y seguridad de ATX tópico para reducir la hemorragia postoperatoria en la artroplastia de rodilla.
Material y métodosUn total de 90 pacientes intervenidos de artroplastia total de rodilla unilateral fueron incluidos en un estudio prospectivo y aleatorizado. Todas las intervenciones fueron llevadas a cabo bajo anestesia espinal, con isquemia preventiva y bajo el mismo régimen postoperatorio. Los pacientes fueron divididos en 3 grupos en función de la administración del ATX: grupo A (n=30) 1g de ATX tópico; grupo B (n=30) 1g de ATX intravenoso, y grupo C o control (n=30), al que no se administró ningún fármaco. Se analizaron los parámetros de pérdida de sangre y débito de drenajes en los 3 grupos.
ResultadosLos resultados mostraron que el descenso del nivel de hemoglobina fue menor en el grupo A (1,95dl) respecto el grupo B (2,25g/dl) y el grupo C (2,96g/dl), p<0,01. Respecto a la hemorragia postoperatoria, también fue inferior en el grupo A (195ml) respecto el grupo B (466ml) y el grupo C (718ml), p<0,01. No hubo diferencias en las complicaciones y la tasa de transfusiones de sangre entre los 3 grupos.
ConclusionesLa aplicación tópica de 1g de ATX reduce significativamente la pérdida de sangre en pacientes intervenidos de artroplastia total de rodilla, en mayor magnitud que la aplicación intravenosa y la no administración.