It is crucial to assess the levels of protection generated by natural infection or SARS-CoV-2 vaccines, mainly in individuals professionally exposed and in vulnerable groups. Measuring T-cell responses may complement antibody tests currently in use as correlates of protection. Our aim was to assess the feasibility of a validated assay of T-cell responses.
MethodsTwenty health-care-workers (HCW) were included. Antibody test to SARS-CoV-2 N and S-proteins in parallel with a commercially available whole-blood-interferon-gamma-release-assay (IGRA) to S-peptides and two detection methods, CLIA and ELISA were determined.
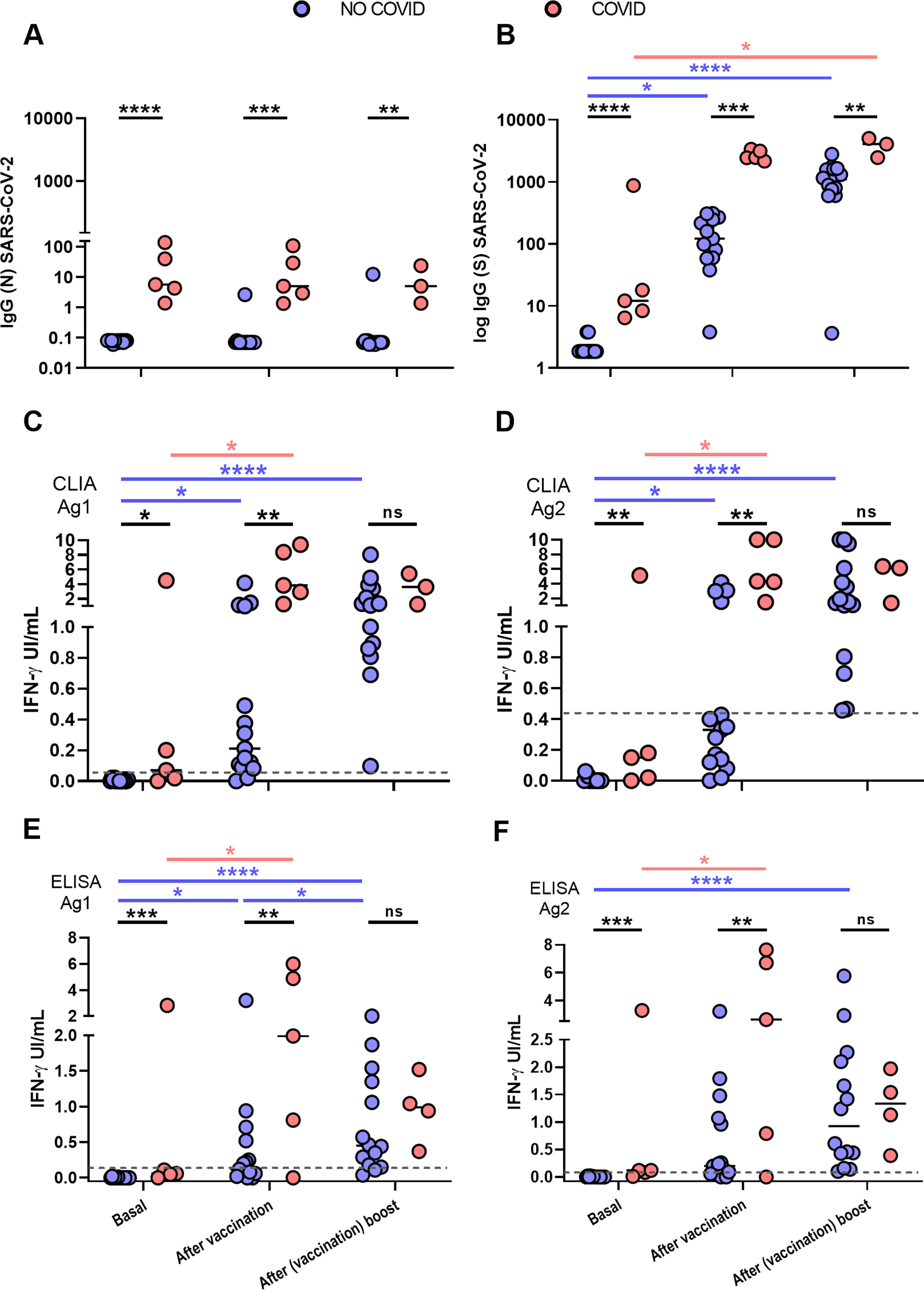
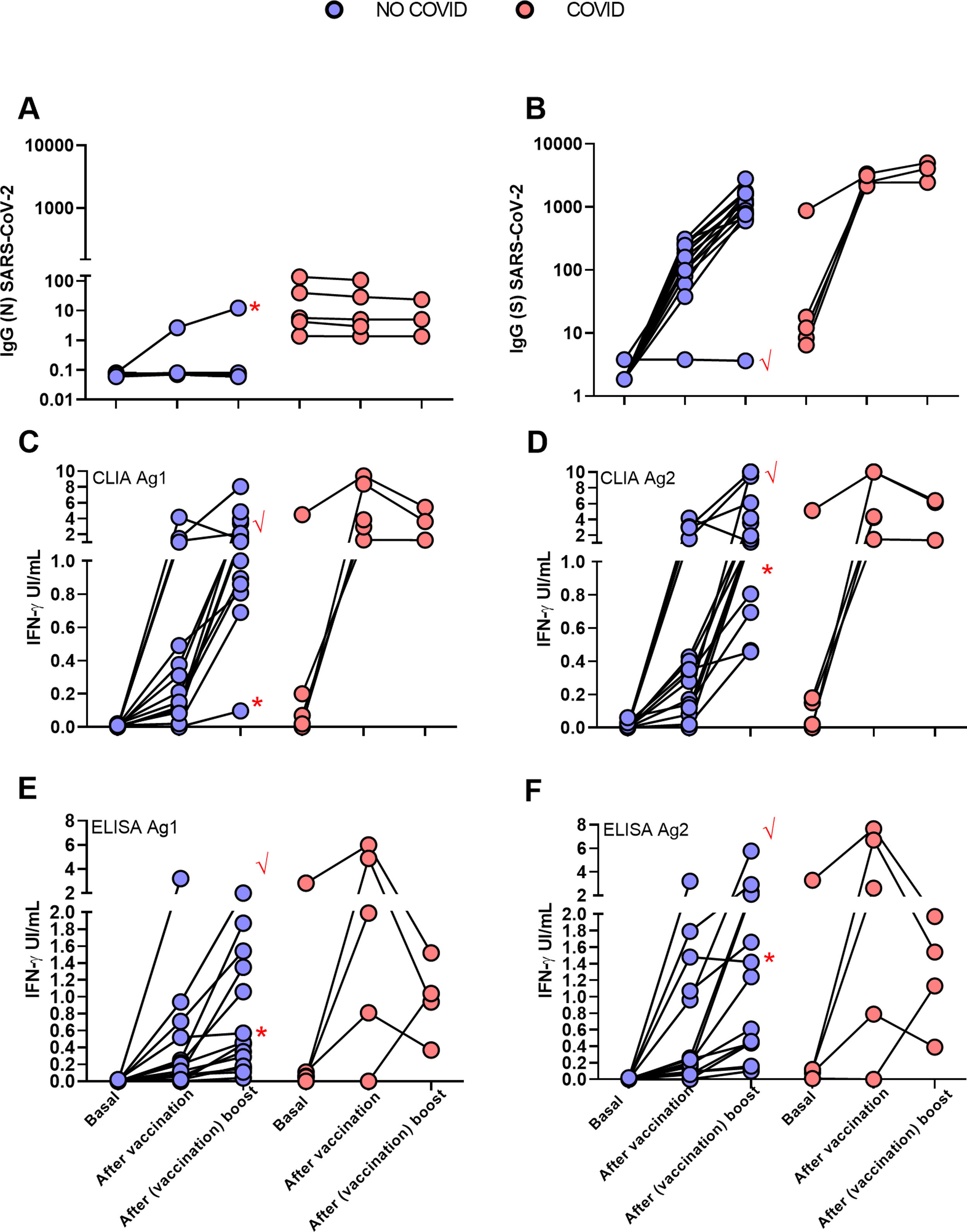
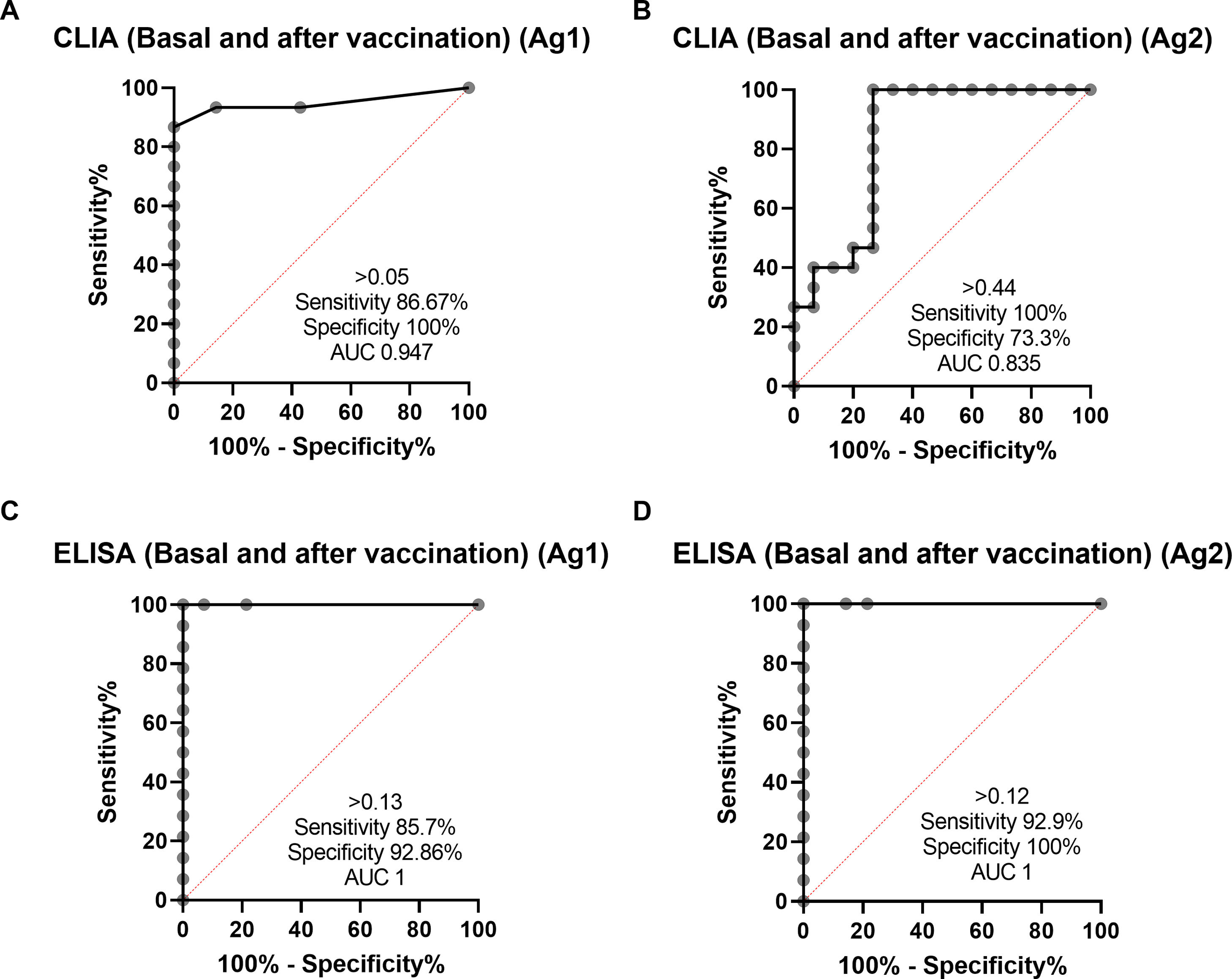
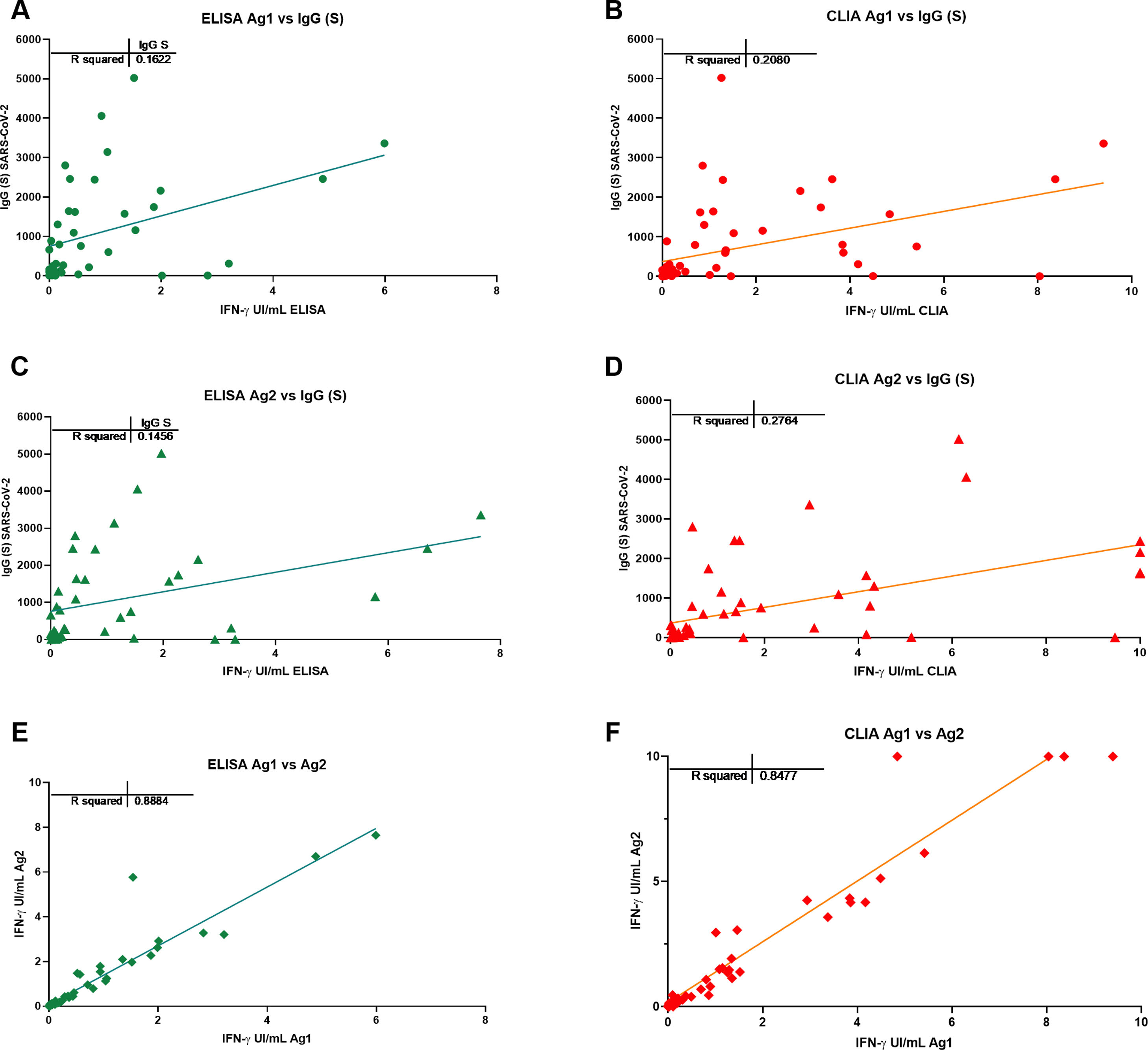
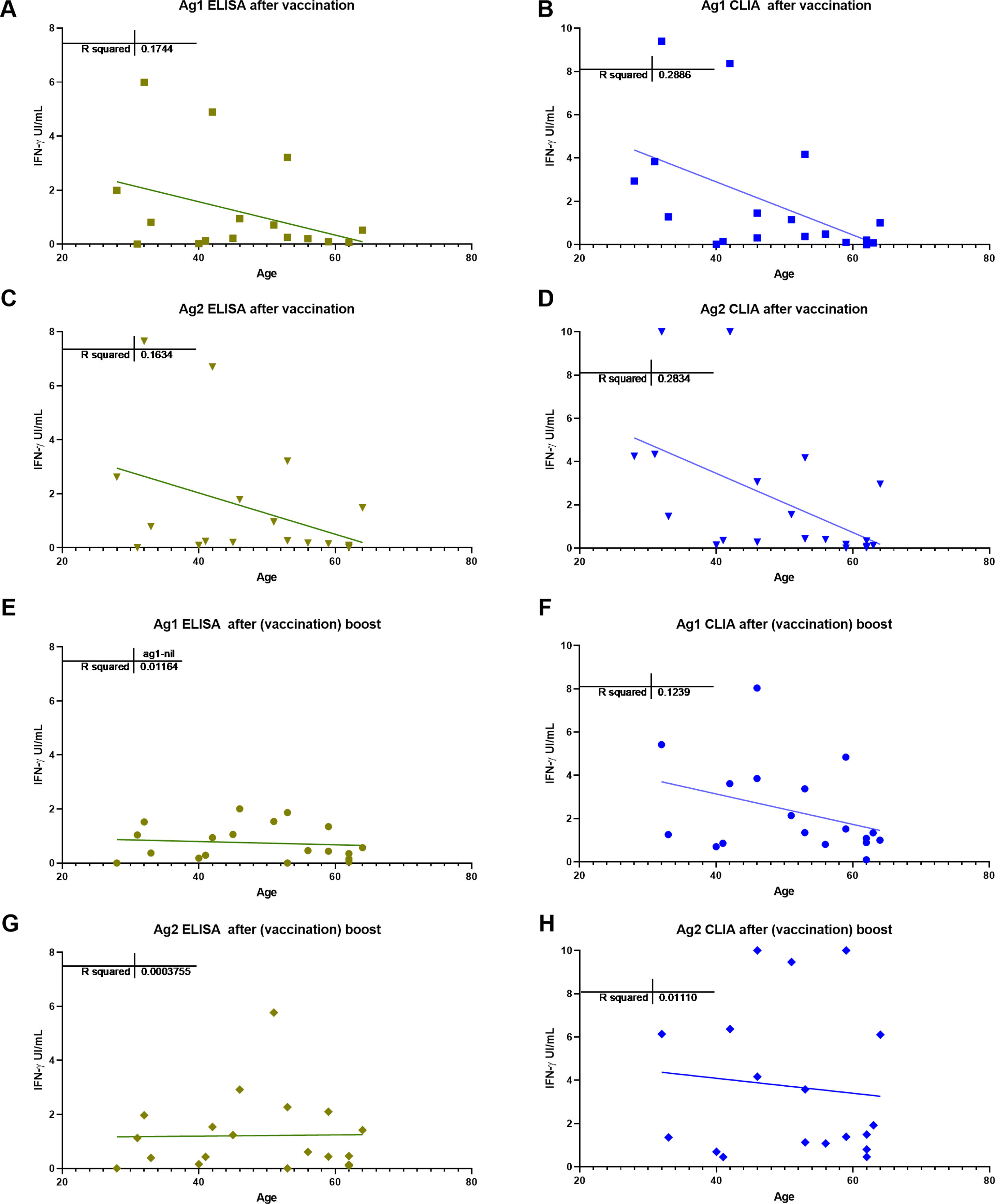
ResultsIGRA test detected T-cell responses in naturally exposed and vaccinated HCW already after first vaccination dose. The correlation by the two detection methods was very high (R>0.8) and sensitivity and specificity ranged between 100 and 86% and 100-73% respectively. Even though there was a very high concordance between specific antibody levels and the IGRA assay in the ability to detect immune response to SARS-CoV-2, there was a relatively low quantitative correlation. In the small group primed by natural infection, one vaccine dose was sufficient to reach immune response plateau. IGRA was positive in one, with Ig(S) antibody negative vaccinated immunosuppressed HCW illustrating another advantage of the IGRA-test.
ConclusionWhole-blood-IGRA-tests amenable to automation and constitutes a promising additional tool for measuring the state of the immune response to SARS-CoV-2; they are applicable to large number of samples and may become a valuable correlate of protection to COVID-19, particularly for vulnerable groups at risk of being re-exposed to infection, as are health-care-workers.
Es fundamental evaluar los niveles de protección inmune en infectados o tras la vacunación frente a SARS-CoV-2. La cuantificación de la respuesta inmune celular T puede complementar la determinación de anticuerpos. Evaluamos la viabilidad de un ensayo comercial validado de respuesta celular T específica frente a SARS-CoV-2.
MétodosSe incluyeron veinte trabajadores sanitarios (TS). Medimos anticuerpos contra las proteínas N y S de SARS-CoV-2 y realizamos el ensayo de liberación de interferón-gamma (IFNγ) en sangre completa (IGRA) frente a péptidos de la proteína S. IFNγ se determinó mediante dos métodos de detección: CLIA y ELISA.
ResultadosIGRA detectó respuesta celular T en TS tanto infectados como vacunados. La correlación de los dos métodos de detección de IFNγ fue muy alta (R>0,8) y la sensibilidad y la especificidad variaron entre 100 y 86% y 100-73% respectivamente. Hubo una concordancia muy alta entre los niveles de anticuerpos específicos y el ensayo IGRA aunque la correlación cuantitativa fue relativamente baja. En el grupo de infectados, una dosis de vacuna fue suficiente para alcanzar el «plateau» de respuesta inmune. IGRA fue claramente positivo en un profesional vacunado inmunosuprimido que presentaba anticuerpos contra la proteína S negativos.
ConclusionesIGRA frente a péptidos de la proteína-S es susceptible de automatización y constituye una herramienta prometedora para medir la respuesta inmune celular frente a SARS-CoV-2; es aplicable a un gran número de muestras y puede servir para valorar la protección, particularmente en los grupos vulnerables en riesgo de volver a exponerse a la infección, como los TS.