Influenza vaccine is recommended for patients with autoimmune inflammatory rheumatic diseases who receive biological therapy. To evaluate if biological therapy impairs immunization after seasonal influenza vaccine.
Material and methodsPatients with inflammatory arthopathies, psoriasis, inflammatory bowel disease or connective tissue diseases who were receiving or were going to initiate biological therapy were included and vaccinated during 2014–2015 influenza season. ELISA was used to measure influenza antigen A and B antibodies, before and after vaccination. Demographic parameters, diagnosis and kind of treatment were recorded and their influence on the final serological status against influenza was studied.
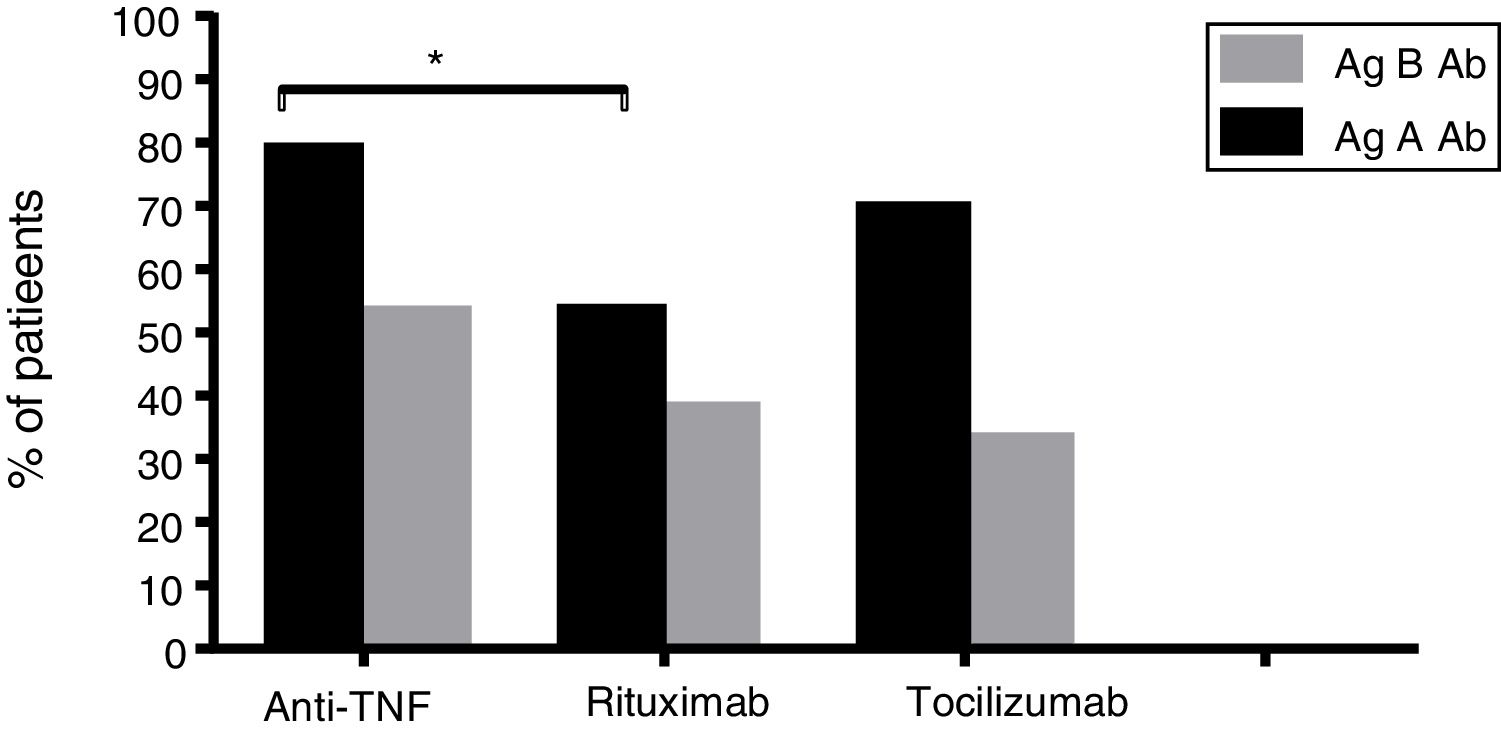
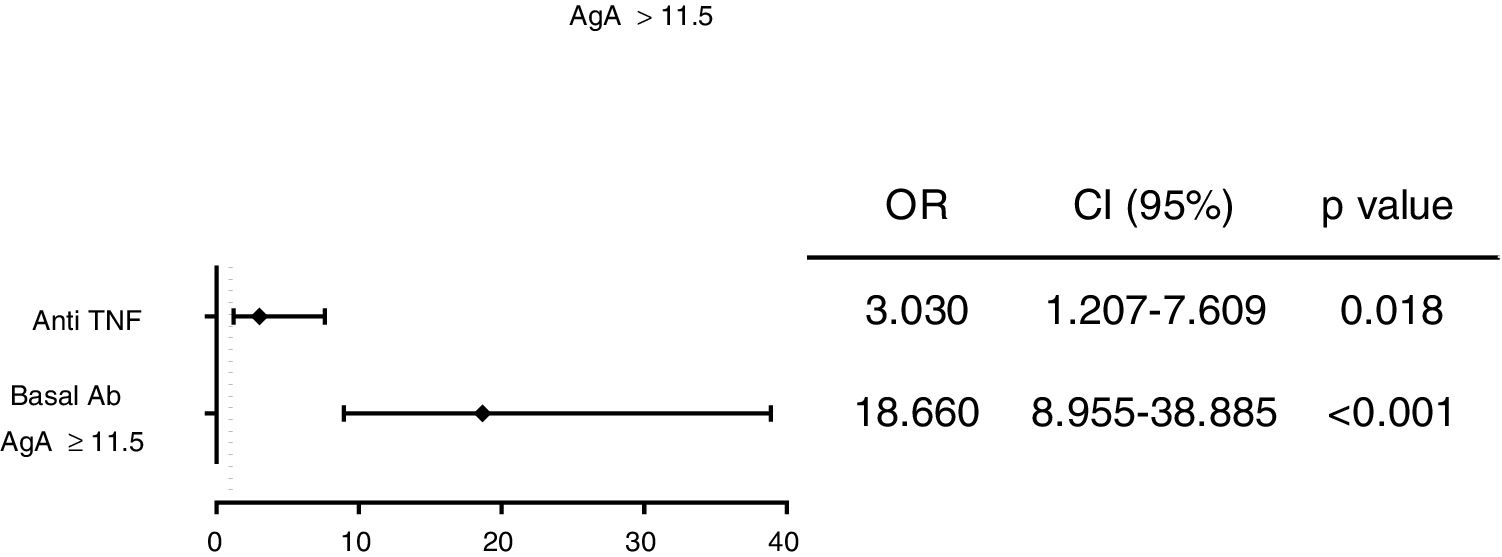
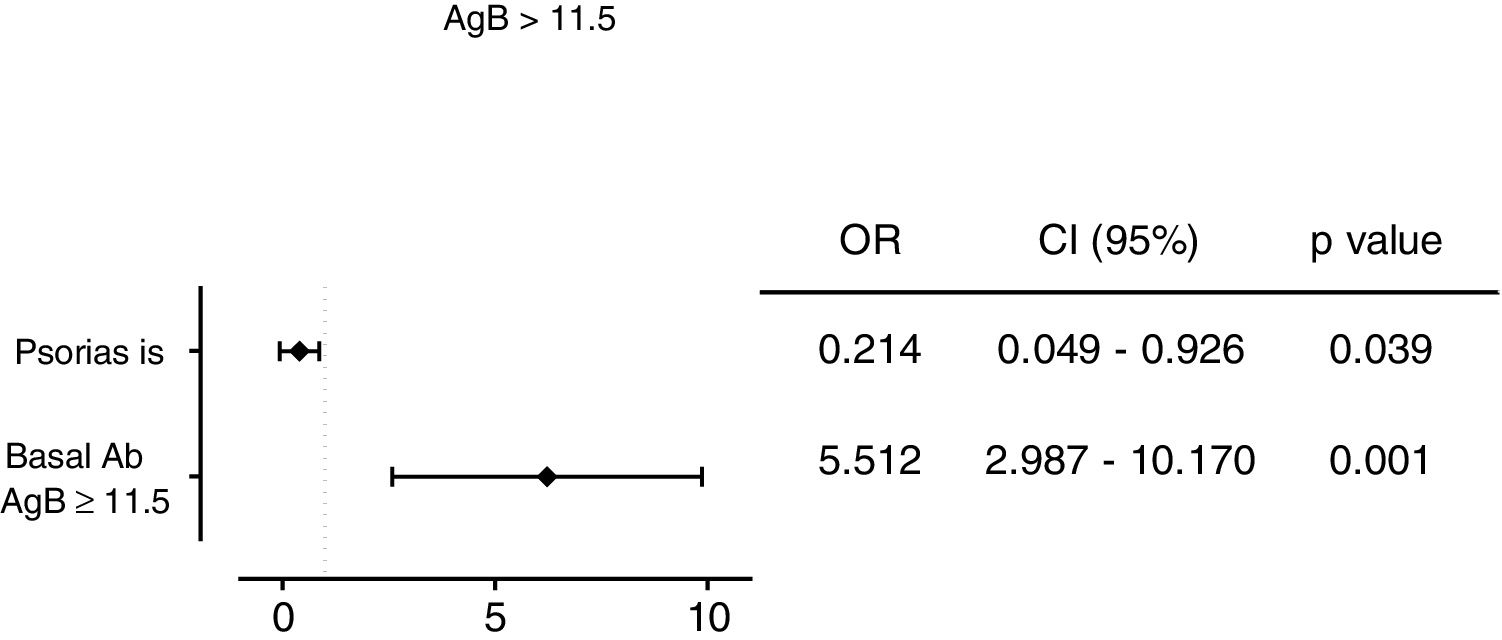
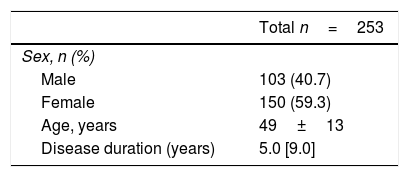
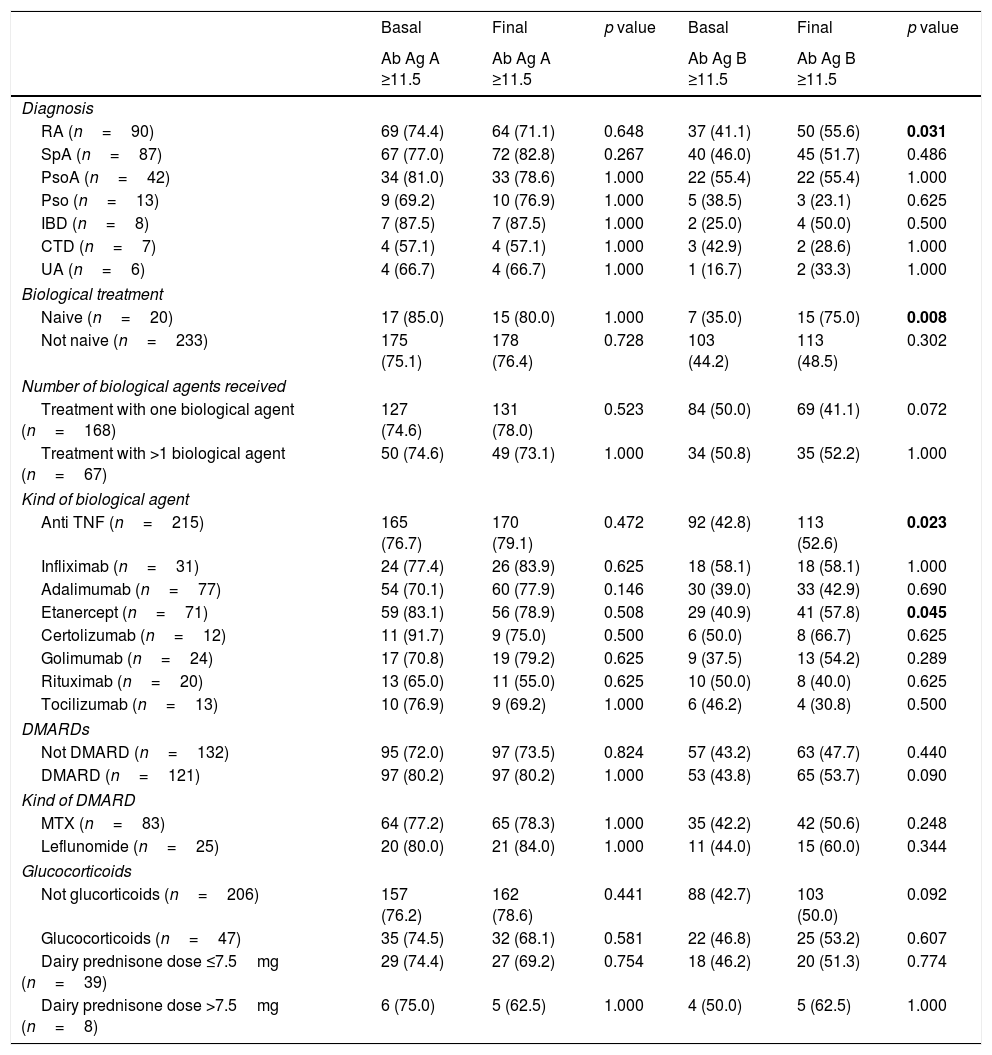
Results253 subjects were analyzed. After vaccination, 77% of participants presented detectable antibodies against antigen A and 50.6% of them had detectable antibodies against antigen B. Final seropositivity rate against antigen B antibodies increased from baseline (50.6% vs 43.5%, p<0.001). Anti-TNF drugs were associated with better response and rituximab with the worst (79.2% vs 55.0% for final seropositivity against antigen A, p=0.020). Vaccine response in the rituximab group tended to improve when the interval between the drug administration and the vaccination was at least 12 weeks (seropositivity rate 80.0% in those with the longer interval vs 25.0% in the other group, p=0.054).
ConclusionsAmong the patients on biological therapy vaccinated against influenza, anti-TNF therapy was identified as a predictive factor of final seropositivity. Rituximab presented a lower rate of final seropositivity, which could be increased with an accurate administration schedule.
La vacunación antigripal está recomendada en pacientes con enfermedades autoinmunes sistémicas que reciben tratamientos biológicos. Evaluar si la terapia biológica puede perjudicar la inmunización después de la administración de la vacuna contra la gripe estacional.
Material y métodosLos pacientes con artropatías inflamatorias, psoriasis, enfermedad inflamatoria intestinal o enfermedades del tejido conectivo, que estaban en tratamiento o que iban a iniciar tratamiento con terapia biológica, fueron incluidos en el estudio y vacunados durante la temporada de influenza 2014-2015. Se utilizó ELISA para medir los anticuerpos contra los antígenosA y B de la gripe, antes y después de la vacunación. Se registraron los datos demográficos, diagnósticos y el tipo de tratamiento y se estudió su influencia sobre el estado serológico final contra la influenza.
ResultadosSe analizaron 253 sujetos. Después de la vacunación, el 77% de los participantes presentaron anticuerpos detectables contra el antígeno A y el 50,6% de ellos tenían anticuerpos detectables contra el antígeno B. La tasa de seropositividad final de anticuerpos contra el antígeno B aumentó desde los valores basales (50,6% frente a 43,5%, p<0,001). Los fármacos anti-TNF se asociaron con la mejor respuesta y rituximab con la peor (79,2% vs. 55,0% para la seropositividad final contra el antígeno A, p=0,020). La respuesta a la vacuna en el grupo de rituximab tuvo tendencia a mejorar cuando el intervalo entre la administración del fármaco y la vacunación fue por lo menos de 12 semanas (tasa de seropositividad del 80,0% en aquellos con el intervalo más largo frente al 25% en el otro grupo, p=0.054).
ConclusionesEntre los pacientes en terapia biológica vacunados contra la influenza, la terapia anti-TNF se identificó como un factor predictivo de la seropositividad final. Rituximab presentó una tasa más baja de seropositividad final, que podría aumentarse con un programa de administración preciso.