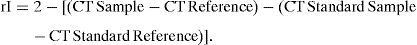The activating MICA/NKG2D interaction is involved in the response of intraepithelial lymphocytes (IELs) in coeliac disease (CD). The aim of this study was to investigate the expression of NKG2D ligands (MICA, MICB), IL-15 and NKG2D receptor in gut mucosa of CD patients, and the correlation with the severity of histological damage.
Patients and methodsIntestinal biopsies from 20 CD patients and five healthy controls were selected. All patients were positive for anti-transglutaminase 2 antibodies and for DQ2 or DQ8. Patients were divided into two groups according to their grade of mucosal impairment: ten each with mild and severe mucosal damage (MMD and SMD, respectively). The expression of proposed genes was determined at mRNA level. MICA expression was also determined by immunohistochemistry.
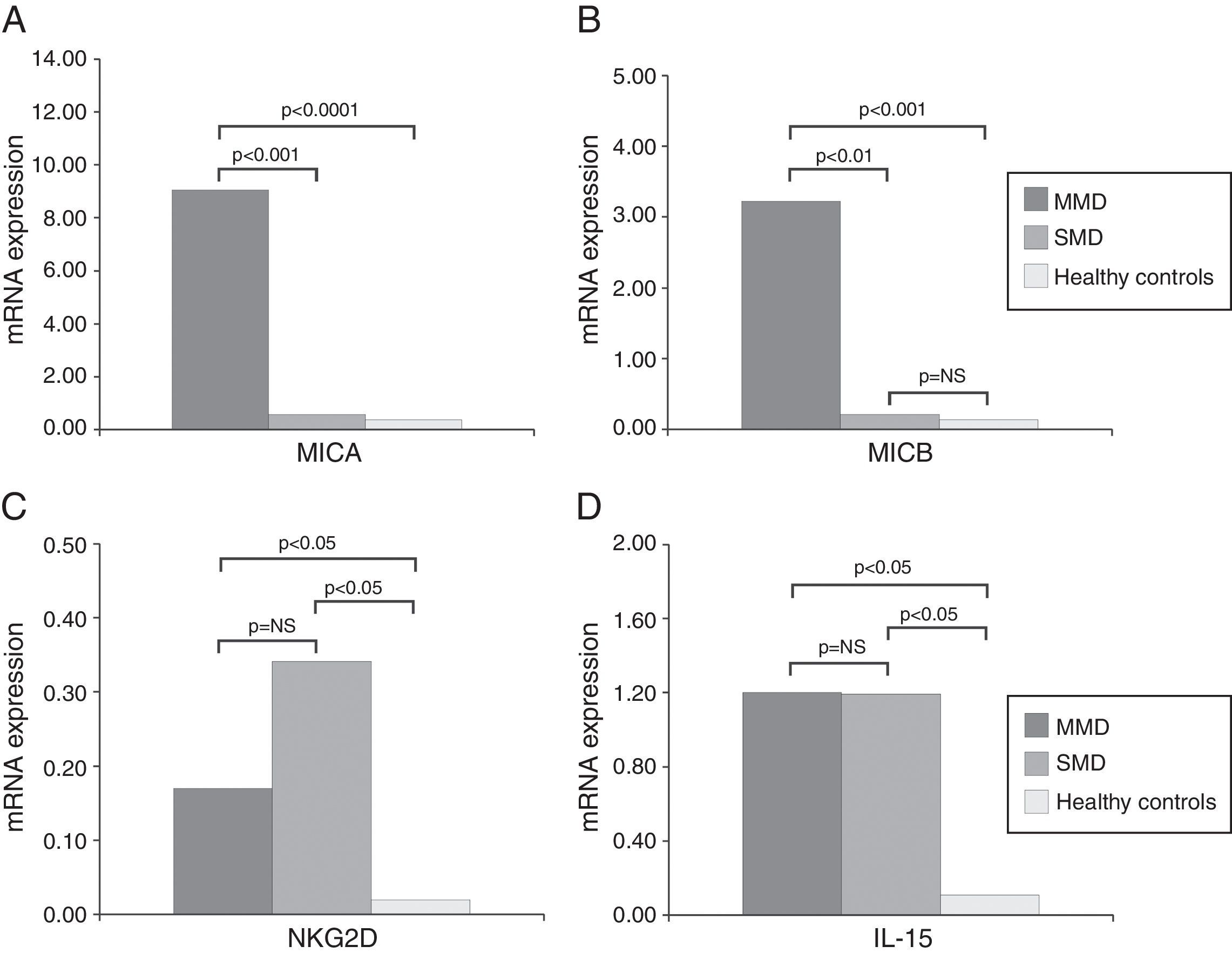
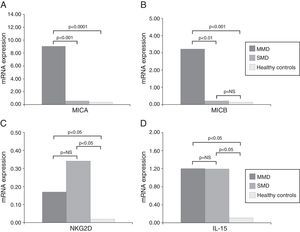
ResultsOverexpression of MICA and MICB was observed in biopsies from coeliac patients compared to healthy controls (P<0.001). Nevertheless, the expression was considerably higher in the group of patients with MMD (P<0.0001) than in those with SMD. The levels of NKG2D receptor and IL-15 were also higher in patients than in controls, but no relationship with the severity of the mucosal lesion was found.
ConclusionsOur results suggest that NKG2D ligands may play an important role during the onset of the inflammatory process in the early stages of the development of coeliac disease.
La interacción de MIC/NKG2D participa en la respuesta de los linfocitos intraepiteliales (LIE) en la enfermedad celíaca (EC). El objetivo de este estudio fue investigar la expresión, en la mucosa intestinal de pacientes con EC, de los ligandos de NKG2D (MICA, MICB), de la IL-15 y del receptor NKG2D, y relacionarla con la severidad del daño.
Pacientes y métodosSeleccionamos biopsias intestinales de 20 pacientes con EC y de cinco controles sanos. Todos los pacientes resultaron positivos para anticuerpos anti-transglutaminasa 2 y para HLA-DQ2 o HLA-DQ8. Los pacientes fueron divididos en dos grupos según el grado de deterioro de la mucosa: diez con daño leve y otros 10 con daño grave (denominados MMD y SMD, respectivamente). En todos ellos se analizó la expresión de MICA, MICB, NKG2D y IL-15 a nivel de ARN mensajero, y en el caso de MICA se completó el estudio analizando su expresión por inmunohistoquímica.
ResultadosSe observó sobreexpresión de MICA y MICB en biopsias de pacientes celíacos en relación con los controles sanos (p<0.001). Sin embargo, la expresión fue notablemente mayor en el grupo de pacientes MMD que en aquellos del grupo SMD (p<0.001). Los niveles de receptor NKG2D y IL-15 también fueron mayores en pacientes que en controles, pero no se encontró relación con la gravedad de la lesión de la mucosa.
ConclusionesNuestros resultados sugieren que los ligandos de NKG2D pueden desempeñar un papel importante durante el inicio del proceso inflamatorio en las primeras etapas del desarrollo de la enfermedad celíaca.
For a long time coeliac disease was thought to be a relatively rare pathology that only appeared in childhood. Now, however, it is recognized as being a very common disease that can be diagnosed at any age.1,2 Among the most typical characteristics of the disease are a strong genetic association with the HLA alleles DQ2 and DQ8 and its onset being precipitated by an environmental factor, namely, gluten.3 Gliadin, the alcohol-soluble fraction of the protein, has been identified as the cause of this intolerance, but the great majority of gluten proteins are also probably toxic in coeliac disease.4,5 These proteins induce an inflammatory process in the intestine, although the process regresses when they are removed from the diet, thereby restoring the proper structure and function of the mucosa.6
The intraepithelial infiltration by lymphocytes is the first stage in the pathogenic process of CD. In the epithelium of an exposed coeliac patient, the populations of CD8+ T cells and γδ+ T cells become more numerous, and both express markers of cellular proliferation and activation. When treatment with a gluten-free diet is instigated, the number of CD8+ lymphocytes returns to normality, whereas intraepithelial lymphocytes (IELs) γδ+ remain permanently increased. The role of such populations is one of the main unresolved matters in the pathogenesis of the CD.7
MHC class I chain-related molecules A and B (MICA and MICB) are homologous with classical HLA-class I but have no role in antigen presentation.8 These are cell surface glycoproteins that are mainly expressed in endothelial, epithelial cells, fibroblasts and activated monocytes. MICA expression is induced by stress situations and is upregulated in infection and tumour transformation. This protein is a ligand for the NKG2D-activating receptor, which is mainly expressed in Natural Killer (NK) and CD8+ T cells. In NK cells, the interaction between MICA and NKG2D induces the cytolytic capacity of these cells, while in CD8+ it acts as a costimulatory signal that complements antigen recognition by T cell receptor (TCR).9
MICA and MICB are both polymorphic, and some variants are associated with coeliac disease. The transmembrane variant MICA 5.1, equivalent to the MICA 008 allele, is associated with atypical forms of CD10 independently of the presence of HLA-DQ2.11 Additionally, the MICB allele 0106 is also overrepresented in patients with atypical forms of CD.12
These ligands are largely unexpressed in most healthy tissues. However, aberrant expression of NKG2DL has been detected in some autoimmune diseases, for example in the prediabetic pancreas islet of NOD mice13 and the synoviocytes of rheumatoid arthritis patients.14 Furthermore, MICA is constitutively expressed at low levels in normal intestinal epithelial cells (IECs). MIC is induced at very high levels on the surface and in the crypts of the small bowel of CD patients.15 It has also been reported that the gliadin-derived peptide 31–49 upregulates MICA expression in epithelial cells as a consequence of IL-15 induction.16 Another study has demonstrated the direct involvement of the MICA-NKG2D interaction in the activation of intraepithelial lymphocytes in response to the direct toxic effect of gluten.17 This activation damages the enterocytes, and could be the initial event that ultimately leads to villous atrophy.
The aim of this study was to investigate the expression of MICA, MICB, NKG2D and IL-15 by RT-PCR and immunochemistry in biopsies of patients with active CD, and to evaluate the association with the severity of the lesion.
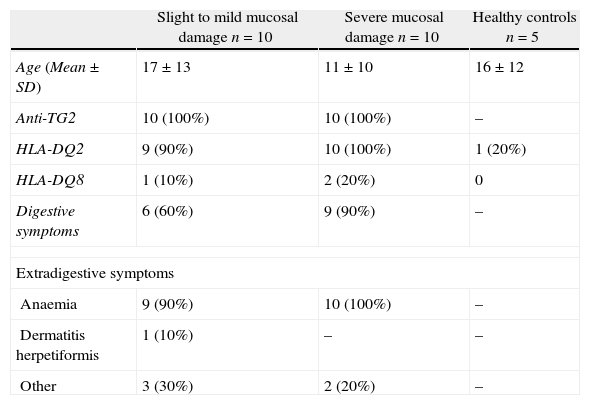
Materials and methodsPatientsThe levels expression of NKG2D, IL-15, MICA and MICB were measured in biopsies from 20 patients diagnosed with coeliac disease. All biopsies were obtained from at least three different sites in the proximal-to-distal duodenum, yielding 72 samples in total. All biopsies were obtained before a gluten-free diet was initiated (Table 1). To ensure a homogeneous group of samples, the patients selected for this study had the same lesion type in all the biopsies collected. Five samples from individuals with no digestive pathology were used as healthy controls.
Clinical and genetic features of coeliac patients and healthy controls.
| Slight to mild mucosal damage n=10 | Severe mucosal damage n=10 | Healthy controls n=5 | |
| Age (Mean±SD) | 17±13 | 11±10 | 16±12 |
| Anti-TG2 | 10 (100%) | 10 (100%) | – |
| HLA-DQ2 | 9 (90%) | 10 (100%) | 1 (20%) |
| HLA-DQ8 | 1 (10%) | 2 (20%) | 0 |
| Digestive symptoms | 6 (60%) | 9 (90%) | – |
| Extradigestive symptoms | |||
| Anaemia | 9 (90%) | 10 (100%) | – |
| Dermatitis herpetiformis | 1 (10%) | – | – |
| Other | 3 (30%) | 2 (20%) | – |
Biopsies from patients were divided into two groups on the basis of the MARSH classification: those with minimal or mild mucosal damage (Marsh I, II and IIIa; MMD) and those with severe mucosal damage (Marsh IIIb and IIIc; SMD). Additionally, all patients and controls were typed for HLA-DQ using the PROTRANS Domino System HLA Celiac Disease kit (PROTRANS GmbH, Ketsch, Germany).
All patients and controls gave informed consent and the study was approved by ethics committee of our Hospital.
Real-time RT-PCRTotal RNA was derived from tissue samples using NucleoSpin RNA II from Macherey-Nagel (Düren, Germany) and reverse-transcribed using the First Strand cDNA Synthesis kit for RT-PCR (AMV) from Roche Diagnostics, GmbH (Mannheim, Germany), following the manufacturer's instructions. The resulting cDNA was amplified with specific primer pairs (Table 2) in duplicate (40 cycles: 95°C for 15s and 60°C for 1min), monitoring the amplification using SYBRGreen chemistry with the ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany). Primers, listed in Table 2, were selected to flank introns whenever possible. Data were analysed using the ΔΔCT method for relative quantification. Briefly, threshold cycles (CTs) for GAPDH, MICA-MICB, NKG2D and IL-15 were determined in duplicate. We chose arbitrary values for healthy donor 1 (HD1) as standard values and calculated the relative increase (rI) in copy number in relation to this standard values according to the formula:
Real-time PCR primers.
| Primers | Sequence |
| GADPH 5′ | 5′-CGG AGT CAA CGG ATT TGG TC-3′ |
| GADPH 3′ | 5′-ATTCATATTGGAACATGTAAACCATGTAG-3′ |
| MICA/B 5′ | 5′-CACCTGCTACATGGAACACAGC-3′ |
| MICA 3′ | 5′-TATGGAAAGTCTGTCCGTTGACTCT-3′ |
| MICB 3′ | 5′-ACATGGAATGTCTGCCAATGATC-3′ |
| NKG2D 5′ | 5′-GAGGTCTCGACACAGCTGG-3′ |
| NKG2D 3′ | 5′-GCAGCAGAAAAAAAATGGAGATG-3′ |
| IL-15 5′ | 5′-CGGCTACCACATCCAAGGAA-3′ |
| IL-15 3′ | 5′-GCTGGAATTACCGCGGCT-3′ |
Similar amplification efficacy for MICA, MICB, NKG2D, IL-15 and GAPDH was determined by analyzing serial cDNA dilutions with values of the slope of the log of the amount of cDNA compared with a CT of <0.1. Amplicons were examined on 3% agarose gels to ensure they were of the correct size.
ImmunohistochemistryA polyclonal serum against the α2 domain of the MICA molecule was generated by immunising rabbits with a synthetic peptide of the 140–160 (MNVRNFLKEDAMKTKTHYHAM) translated sequence of MICA, as described previously.18 This serum, known as s8p, was purified on an AFFI-T gel affinity column (KE-MEN-TEC, Copenhagen, Denmark), following the manufacturer's instructions, and then tested in parallel with a negative control of preimmune rabbit serum. For immunohistochemistry staining, 5-μm-thick sections of small gut biopsies from paraffin blocks were cut and mounted on polylysine-coated slides. Deparaffinised samples were blocked with 10% normal human serum in PBS for 30min at room temperature to eliminate non-specific bindings. Sections were incubated with polyclonal serum anti-MICA s8p, for 1h at room temperature in a humid chamber. Antibody binding was detected using the peroxidase EnVision System (Dako, CA, USA). In negative controls, the primary antibody was omitted or replaced by a preimmune rabbit serum at the same dilution.
Statistical analysisUnivariate analyses were used to describe the characteristics of the study sample. Student's t test was used to compare differences between the means of continuous variables, statistical significance being concluded for values of p<0.05.
ResultsThe primary aim of our study was to analyse the expression of MICA in biopsies of patients diagnosed with CD by real-time PCR. First, patients clearly showed increased expression levels than healthy controls (mean value 4.96 vs. 0.16; p<0.001). When we analysed data from CD patients with mild mucosal damage, we found higher mean levels of MICA than those with severe damage (9.04 vs. 0.34; p<0.001). MMD group had increased expression level of MICA mRNA comparing healthy controls (56 times greater; 9.04 vs. 0.16; p<0.0001) (Fig. 1A). Similarly, the level of expression of MICB was also higher in the same group of biopsies. The average value in MMD patients was 3.22 while the biopsies of SMD patients had a mean value of 0.14 (p<0.001). This implies that the expression level of MICB is 27 times that of samples obtained from CD patients with early-stage lesions. We also found that the expression of MICB was 46 times that in MMD (3.22 vs. 0.07; p<0.01) and only 2 times that in the SMD group (0.14 vs. 0.07; p=NS) compared with healthy control group in both cases (Fig. 1B).
Nevertheless, in the case of NKG2D, the mean level of expression in the MMD group of biopsies was slightly lower than that of the SMD patients (0.17 vs. 0.34; p=NS), although the mean level of expression of this receptor was clearly stronger in both groups of patients than in healthy controls (0.003; p<0.05) (Fig. 1C). These results could reflect the greater number of lymphocytes expressing NKG2D, which infiltrate the mucosa in CD patients, but it is nevertheless clear that there were no associations with the degree of damage.
Although IL-15 is known to be a poorly secreted cytokine, we were able to measure mRNA levels by RT-PCR from CD patients (Fig. 1). Both MMD and SMD groups showed similar mean levels of IL-15 expression; these were significantly higher on average than in healthy controls (MMD=1.20, SMD=1.19, HC=0.07; p<0.05) (Fig. 1D).
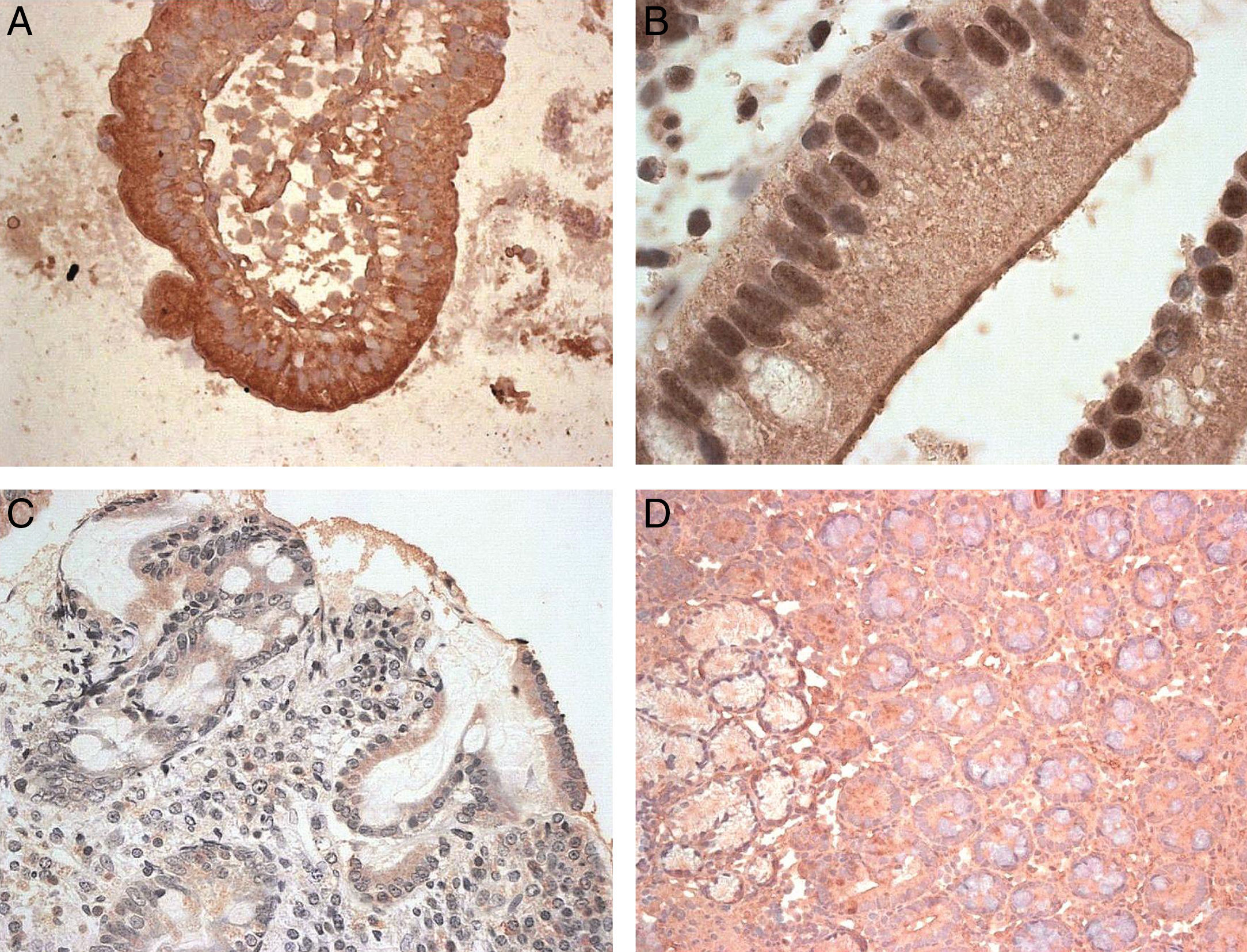
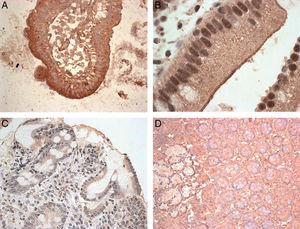
After analysing the expression of these molecules at the mRNA level, the next step was to identify the MICA protein in biopsies of CD patients. The immunohistochemical analysis of the samples showed a positive epithelial stain in all patients, although there were striking differences in the intensity and pattern of staining between the two groups of samples (Fig. 2). First, biopsies from the MMD group showed an intense expression of MICA that was mainly localised in enterocytes, with apical and intracellular distribution. In samples from the SMD group, less intense MICA staining, mainly distributed in the cytoplasm, was observed. Moreover, there was a positive stain of mononuclear cells within the lamina propia in both groups of samples. Healthy control samples exhibited weak MICA staining in the cytoplasm of the epithelial cells. These results are consistent with the high level of expression of MICA revealed by RT-PCR in samples from patients with mild mucosal damage, and suggest that the expression of this protein may be associated with the initial phases of the lesion.
Expression of MICA in gut biopsies of CD patients.
Footnote: A. MICA high-intensity stain in an intestinal villus from a patient with mild mucosal damage. B. Detailed image of enterocytes with an apical MICA expression pattern. C. Gut mucosa with severe damage. The level of expression of MICA is clearly lower than that in the MMD image. D. Normal gut mucosa.
It has previously been reported that, in coeliac disease (CD), intraepithelial intestinal lymphocytes (IELs) express high levels of NKG2D and expand massively under strong exposure to IL-15 in the epithelial compartment.19 IL-15 is overexpressed in active CD mucosa, where its level is correlated with the degree of tissue damage, as revealed by morphometric analysis, and progressively decreased upon instigation of a gluten-free diet.20 Enterocytes and lamina propia mononuclear (LPM) cells are the main source of this cytokine. IL-15 elicits a series of biochemical changes in the NKG2D signalling pathway, converting cytotoxic T cells (CTL) into lymphocyte activating killer (LAK) cells that contribute to tissue damage in coeliac patients.21 Additionally, the NKG2D ligands MICA and MICB are overexpressed in gut mucosa in response to these immunological changes, in accordance with previous reports.15,17 Our study also confirms that levels of MICA and MICB mRNA are higher in biopsies of CD patients, although, unexpectedly, we found that the levels were inversely correlated with the severity of mucosal damage.
Customarily, intraepithelial infiltration by T CD8+ cells has been considered to be secondary to the activation of T CD4+ cells in the lamina propia because no intraepithelial lymphocytes are restricted by gliadin.7 However, these infiltrates are not present in other intestinal disorders associated with inflammation of the lamina propia, such as Crohn's disease and other autoimmune enteropathies. This finding suggests that the activation of the CD4+ T cells of the lamina propia does not explain the expansion of intraepithelial lymphocytes in coeliac disease.7 The immune response of the T cells may be directed not only against peptides, but possibly also towards recognising damaged cells that express molecules induced by cellular stress (MIC and HLA-E) and gamma-interferon.6 It has been suggested that these alterations of the intestinal epithelium can occur in coeliac disease. MIC and HLA-E are recognised by the NK cell receptors NKG2D and CD94 present in the intraepithelial lymphocytes and whose expression is strongly induced by IL-15.8,9 Epithelial damage could be caused by the deregulation of this system in the presence of high concentrations of IL-15. The induction of these activating receptors could lead to the uncontrolled activation of the intraepithelial lymphocytes and, ultimately, to villous atrophy.21 In fact, IL-15, which enables NKG2D-mediated lymphokine killer activity in CTLs, cooperates with NKG2D to induce cytosolic phospholipase A(2) (cPLA2) activation and arachidonic acid release, which leads to tissue inflammation.22 Our results suggest that the levels of IL-15, determined by RT-PCR, were not closely correlated with the degree of mucosal damage, but were clearly higher than in healthy controls, and were maintained at this high level until the final stages of mucosal impairment. IL-15 may be released by enterocytes as a consequence of proteolytic shedding during the course of CD.23 The blockade of the activity of this cytokine could benefit coeliac patients, as has been demonstrated in animal models.24
In the present study we have analysed the expression of MIC molecules in samples from the mucosa of CD patients with varying degrees of damage. We found the highest levels of MICA and MICB in samples with Marsh I, II and IIIa. The corresponding level of expression was progressively lower in samples with Marsh IIIb and IIIc type lesions. These findings suggest that the increased expression of MIC could be an initial phenomenon related to gluten toxicity and the onset of mucosal damage. In fact, the pathological changes in the enterocytes and the extent of the lesion are inversely correlated with the levels of expression of NKG2D ligands. The increases in the level of expression of MIC molecules on the surface of the enterocytes during the initial stage of coeliac disease make them a target for Tαβ/CD8+ lymphocytes, which infiltrate the epithelium. The recognition of MICA and MICB by the NKG2D receptor, whose expression is induced by IL-15, acts as a signal for IELs, inducing its activation and leading to damage of enterocytes, thereby causing villous atrophy. The downregulation of MIC expression during the course of mucosal damage may act as a control mechanism for IEL activation, which is involved in the epithelial damage. Additionally, we found that MICA was also expressed in mononuclear cells, which infiltrate the lamina propia. Under these circumstances, the MICA/NKG2D interaction could act as an additional costimulatory signal for NKG2D-positive CD4 T cells. Although we have not analysed the expression of NKG2D in CD4 T lymphocytes, previous studies have shown this molecule to be habitually present in these cells in some autoimmune diseases25,26 and in ageing people27.
In conclusion, it is clear that the interaction between NKG2D and MICA/B plays an important role in the pathogenesis of CD, and that this is probably more important in the initial phases of the disease. Blockade of the MIC/NKG2D interaction during the early stages of mucosal injury may prevent the progression of tissue damage and the establishment of clinically significant coeliac disease.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
Financial disclosureThis work was supported by grants from Instituto de Salud “Carlos III” FIS PI08/0566 and PI12/02587, and by “Fondos FEDER” from European Union
Conflicts of interestThe authors declare no conflict of interest.