As reported by WHO in its most recent report, H5N1 influenza virus affects mostly children. Differences in morbidity depending on the age could be explained, at least in part, by the differences in the immune response between children and adults. The main objective of the study was to evaluate the effect of H5N1 influenza haemagglutinin on the cytokine secretion profiles of peripheral blood mononuclear cells (PBMCs) from both children and adult healthy donors.
Materials and methodsWe compared the profiles of 19 cytokines and chemokines released after exposure to PBMCs obtained from 30 healthy children and 30 healthy adults to a recombinant glycosylated H5 haemagglutinin after 24h of culture with this antigen, compared to untreated control cells.
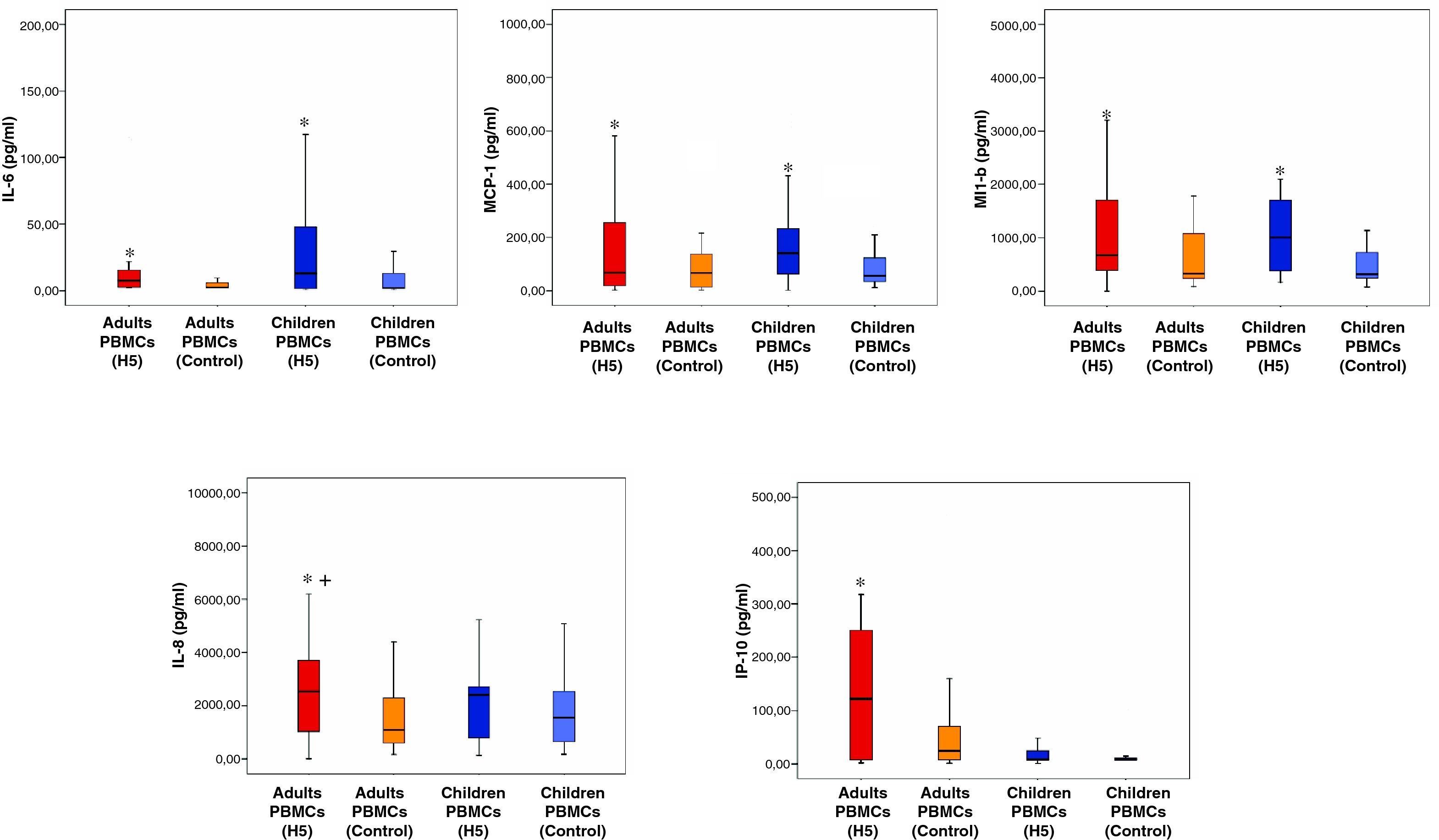
ResultsDonors had not been exposed previously to the virus, as evidenced by the absence of haemagglutination inhibition activity in their plasma samples. Direct contact of PBMCs from both children and adults with the haemagglutinin of H5N1 virus induced increased levels of IL-6, MCP-1 and MIP-1β in the culture supernatants compared to control. Supernatants of adult PBMCs also showed increased levels of IL-8 and IP-10.
ConclusionsThe results of our study supports the idea that, in the absence of protective immunity, the haemagglutinin of the H5N1 virus is able to induce the release of pro-inflammatory mediators by direct contact with PBMCs. Some of these mediators (IL-6, MCP-1 and MIP-1β) are induced regardless of age and have been previously reported to be associated with a poor control of viral replication in severe patients and animal models.
Informes recientes de la Organización Mundial de la salud demuestran que el virus gripal H5N1 afecta principalmente a niños. La morbilidad dependiendo de la edad podría explicarse, al menos en parte, por una respuesta inmune diferente en los niños y los adultos. El objetivo de este estudio fue evaluar el efecto de la hemaglutinina del virus H5N1 en la secreción de citocinas en células mononucleares de sangre periférica (CMSP) en donantes adultos y niños.
Material y métodosEn este estudio comparamos los perfiles de secreción de 19 citocinas y quimiocinas en cultivos de CMSP de 30 niños y 30 adultos sanos 24 horas después de su estimulación con la hemaglutinina glicosilada H5, comparándolo con células control sin tratar.
ResultadosLa ausencia de actividad de inhibición de la hemaglutinación demostró que ninguno de los donantes habían estado en contacto previo con el virus. El contacto directo de las CMSP con la hemaglutinina del virus H5N1 indujo un incremento respecto a los controles de los niveles de IL-6, MCP-1 y MIP-1β, tanto en niños como en adultos. Además, los sobrenadantes de cultivo de CMSP de adultos tenían mayores niveles de IL-8 e IP-10.
ConclusionesLos resultados de nuestro estudio apoyan la idea de que, en ausencia de inmunidad protectora, el contacto directo entre la hemaglutinina del virus y las CMSP es capaz de inducir la liberación mediadores pro-inflamatorios. Algunos de estos mediadores (IL-6, MCP-1 y MIP-1β) se secretan de forma independiente a la edad, y se han relacionado con anterioridad con un mal control de la replicación del virus en pacientes graves o en modelos animales.
H5N1 influenza virus is endemic in poultry in South East Asia and Africa and possesses the ability to infect humans causing severe diseases. Human infection by H5N1 influenza virus is a major challenge for the medical community due to the possibility to generate strains with pandemic potential. As reported by WHO, H5N1 influenza virus affects mostly to children. During 2009, the median age of the reported cases with laboratory confirmed infection by H5N1 was 5 years,1 with cases over 40 year old underrepresented.2 Differences in morbidity depending on the age could be explained, at least in part, by the existent differences in the immune response between children and adults.3 Severe H5N1 infection has been associated with the presence of hypercytokinemia.4 H5N1 viruses induce the secretion of cytokine and chemokine by primary alveolar and bronchial epithelial cells and macrophages more potently than other influenza strains.5,6 The exact role that hypercytokinemia plays in disease pathogenesis is unkown, but it is thought to be related with the haemophagocytic syndrome observed in this patients7 and it parallels uncontrolled viral replication.4 Influenza haemagglutinin (HA) is a glycoprotein present in the surface of the virus which binds to sialic acids in the outer plasmatic membrane, a key process to initiate the internalization of the virus into the cell. HA is a major antigen that initiates humoral immunity against infection. This protein has an important genetic variability. There are at least 16 different HA labelled H1–H16.8 This genetic variability is one of the major contributors of the seasonal epidemics (caused by minor antigenic variations) and pandemics (caused by major antigenic variations). Baculovirus-expressed heamagglutinins are a good alternative for the study of the immune response against influenza7 and are being used for the development of vaccines.9 By studying specific cytokine secretion profiles induced by H5 haemagglutinin in peripheral blood mononuclear cells from both children and adults we intended to describe the influence of age on the secretion of soluble immune mediators induced by this key antigen of the virus.
Materials and methodsExperimental design, samples and donors30 adult healthy blood donors were recruited in the “Centro de Hemodonacion y Hemoterapia de Castilla y Leon”, Valladolid, Spain from February to March 2009, before the emergence of the new H1N1 pandemic strain. 30 paediatric donors with no pathological antecedents needing of pre-operatory hemogram and biochemistry analysis before undergoing surgery (umbilical hernia, tympanic surgery) were recruited in the Clinical Analysis Service in “Hospital Clínico Universitario” in the same city. The study protocol was approved by the Committee on ethics in Clinical Research of our hospital. Informed consent was obtained directly from each patient or their legal representative.
Cell cultures and antigens10ml of blood were collected in EDTA tubes and immediately centrifuged in Ficoll gradient (Lymphoprep, Axix-Shield) in the Infection, Immunity and Genomics Lab, Hospital Clínico Universitario de Valladolid. Peripheral blood mononuclear cells (PBMCs) were recovered and seeded (200,000 cells in 250μl of complete RPMI medium with antibiotics, glutamine and 10% of foetal calf serum). PBMCs from each donor were treated for 24h with glycosylated recombinant haemagglutinins from three influenza A viruses (A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/2005 (H3N2), and A/Vietnam/1203/04 (H5N1)), as previously described (300ng/ml).7 The haemagglutinins, abbreviated as H1, H3, and H5 respectively; were purchased to Protein Sciences. A well with untreated cells was included as control for each donor. Culture supernatants were stored at −80°C until cytokine and chemokine evaluation.
Immune mediatorsChemokine and cytokine levels were evaluated using the multiplex Biorad© 17 plex assay following manufacturers’ instructions. This system allows for quantitative measurement of 17 different chemokines, cytokines, growth-factors and immune mediators while consuming a small amount of biological material. Furthermore, this system has good representation of analytes for inflammatory cytokines, anti-inflammatory cytokines, Th1 cytokines, Th2 cytokines, Th17 cytokines and chemokines, allowing for the testing of differential levels of regulatory cytokines in the serum of patients. Mediators profiled were MCP-1, MIP-1β, GM-CSF, G-CSF, IL-8, IL-17, IL-6, IL-13, IL-4, IL-5, IL-1β, IFN-γ, TNF-α, IL-12p70, IL-2, IL-10, and IL-7. Enzyme-Linked ImmunoSorbent Assay (ELISA) kits were employed to measure IP-10 (Quantikine, R&D) and IFN-α (VeriKine, Thermo).
Haemagglutination inhibition (HI) assaysHI were performed on a 100μl aliquot of plasma at the National Influenza Center in Valladolid, Spain. The sera was treated with Receptor-Destroying Enzyme (RDE) of V. cholerae by diluting one part serum with three parts enzyme and were incubated overnight in a 37°C water bath. The enzyme was inactivated by a 30-min incubation at 56°C followed by the addition of six parts 0.85% physiological saline for a final dilution of 1/10. HI assays were performed in V-bottom 96-well microtiter plates (Corning Costar Co., Cambridge, MA, USA) with 0.5% turkey erythrocytes, as previously described,10 using a WHO Influenza A(H5) reagents for HAI test.
Statistical analysisImmune mediators’ concentration in culture supernatants was compared between H1, H3, H5 and control by using the Friedman Test. If the Friedman Test indicated a significant difference in the level of a particular mediator, statistical significance was further assessed by using the Wilcoxon test (P<0.05 considered statistically significant). Levels of mediators were compared between adults and children culture supernatants by using the U-Mann-Whitney test. Association between mediators’ levels were studied by using the Spearman-Karber test.
ResultsClinical, serologic and demographic dataThe participants in this study had no received previous vaccination against influenza. They had no signs or symptoms of inflammatory or infectious disease at the time of sample collection, exhibiting a normal hemogram. Adult donors were 39.0 (9.1) yr old (mean, SD) and the paediatric group was 5.4 (2.9) yr old (mean, SD). Sex distribution was (M/F) (25/5) in the adult group and (14/16) in the paediatric group.
The HI assay revealed the absence of antibodies against the H5N1 in the patient plasma.
Immune mediatorsIL-6, IL-8, MCP-1, MIP-1b and IP-10 were the only mediators secreted in detectable levels in the culture supernatants (immune mediators levels are shown in Table 1 and Fig. 1). Concentration of the remaining mediators studied was below the limit of detection of the assays employed. No differences were found between adults and children in the levels of none of the mediators studied in the culture supernatants with none of the hemaglutinins evaluated, except for a chemokine, IP-10, which concentration was higher in adults than in children.
Immune mediators’ levels in culture supernatants after challenge with H1, H3 and H5 recombinant hemagglutinins. Results are expressed as median [interquartile range] in pg/ml. n.s: non significant.
| H1 | H3 | H5 | NC | H1vsH3 | H1vsH5 | H3vsH5 | |
| ADULTS | |||||||
| IL-6 | 2,660 [3,17] | 3,730 [9,97] | 7,440* [13,40] | 2,4600 [3,89] | n.s | 0.004 | n.s |
| IL-8 | 1.143,600 [1.938,04] | 1.366,860 [1.719,17] | 2.540,300* [3.023,72] | 1.090,1400 [1.944,59] | n.s | 0.028 | 0.003 |
| MCP-1 | 52,200* [235,61] | 81,920* [210,00] | 66,740* [273,19] | 66,0100 [128,77] | 0.013 | 0.000 | n.s |
| MIP-1b | 384,670* [968,21] | 488,960** [1.347,69] | 672,310* [1.321,62] | 321,6300 [1.050,07] | n.s | 0.062 | n.s |
| IP-10 | 154,607* [791,95] | 311,118* [759,29] | 122,053* [242,59] | 24,1347 [65,42] | n.s | 0.033 | 0.003 |
| CHILDREN | |||||||
| IL-6 | 4,270 [10,99] | 5,375 [24,12] | 12,925* [46,70] | 1,8450 [13,04] | n.s | 0.035 | 0.076 |
| IL-8 | 1.261,080 [2.077,04] | 1.574,000 [1.732,98] | 2.414,545 [2.030,94] | 1.557,5000 [2.004,36] | n.s | 0,072 | n.s |
| MCP-1 | 87,220* [94,86] | 107,705* [107,46] | 139,595* [174,08] | 55,2650 [92,90] | 0.001 | 0.000 | 0.023 |
| MIP-1b | 456,130 [555,69] | 555,005* [1.154,81] | 1.007,810* [1.154,81] | 313,0400 [497,70] | 0.008 | 0.001 | 0.073 |
| IP-10 | 9,297* [63,43] | 14,785* [50,36] | 7,800 [16,99] | 7,8000 [3,34] | n.s | 0.008 | 0.007 |
When paired comparisons were performed for each donor for the different conditions assayed against the control, H1, H3 and H5 were able to induce the secretion of MCP-1 over control levels in both age groups. H5 treated wells showed higher levels of IL-6 and MIP-1β in the supernatants than control ones, also in both groups. Interestingly, H5 was the only haemagglutinin able to induce the secretion of IL-6.
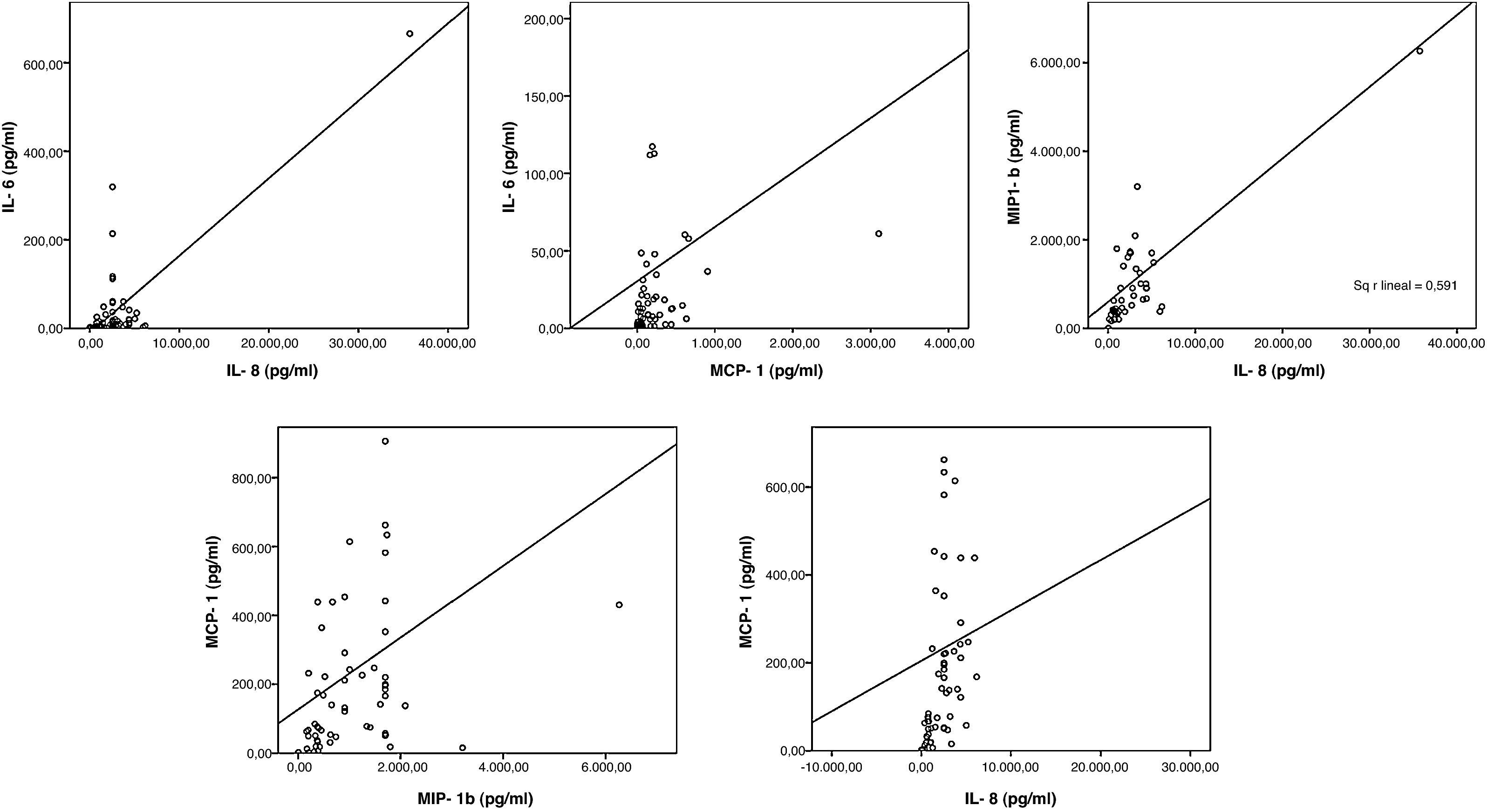
Adult PBMCs treated with H5 (but not children's ones) secreted IP-10 and IL-8 in significant higher levels than control. As for IL-6, secretion of IL-8 was exclusively observed in the PBMCs treated with H5 haemagglutinin but no in those treated with H1 or H3. Remarkably, the levels of IL-6, MCP-1 and MIP-1β and IL-8 in culture supernatants of PBMCs treated with H5 showed a positive correlation (p<0.05) (Fig. 2).
DiscussionAs expected, none of the patients studied had experimented previous contacts with H5N1, as demonstrates the absence of antibodies against this virus in the patients plasma.
Secretion of MCP-1, IL-6 and MIP-1β by both children and adult PBMCs evidences that they constitute a preserved mechanism of the immune response to H5N1 following an initial contact with haemagglutinin, protein which mediates virus binding to the cells. Elevation of MCP-1 and MIP-1β is commonly observed in the context of respiratory viriasis, and it is linked to the development of the innate immunity against viruses.11–13 IL-6 is elevated in the context of airway inflammatory diseases, such as asthma, and is secreted also in severe disease caused by other respiratory viruses.11,12 IL-6 is associated with the development of Th17 responses, recently described to play contradictory roles during influenza infection.14,15 Interestingly, IL-6 and MCP-1 levels in plasma have shown to correlate with viral load in pharynx in severe H5N1 disease.4 Based on the result of this study, the hyper-production of IL-6 observed during H5N1 infection can be caused, at least in part by the haemagglutinin of this virus, ability not showed by haemagglutinins of other influenza strains. MIP-1 has been observed to parallel uncontrolled viral replication in mice models.16 Age-independent secretion induction of MCP-1, IL-6 and MIP-1β by H5 evidence that other factors such as the existence of crossed-immunity against the virus in adults as consequence of previous and repeated contacts with other influenza strains could explain the absence/mild disease observed after H5N1 infection in this group of age.17 On the other hand, in children, having a limited/absent presence of protective antibodies against influenza viruses, the virus would do induce a prominent cytokine secretion, which in turn could mediate tissue inflammatory damage. This could contribute to the severe respiratory and systemic disease observed in infants and adolescents infected by H5N1.18
IL-8 and IP-10 play an important role in the innate defence against respiratory viruses.11,12 The specific induction of IL-8 and IP-10 in adult PBMCs by H5 could represent a sort of protective mechanism, since severity is focused in younger ages in this disease. The positive correlation observed in the immune mediators levels indicate that they are secreted in a coordinated manner in response to the challenge with this protein, in both adults and children.
The results of our study supports the idea that, in absence of protective immunity, as occurs in young children, the haemagglutinin of the H5N1 virus is able to induce the release of pro-inflammatory mediators by direct contact with PBMCs. Some of these mediators (IL-6, MCP-1 and MIP-1β) are induced in an age-independent manner and have been previously reported to be associated with a poor control of viral replication in severe patients and animal models.
Author contributionsThe study was designed and analyzed by R. Almansa, J.F. Bermejo-Martín and R. Ortiz de Lejarazu; laboratory studies were performed by R. Almansa, L. Rico and V. Iglesias; clinical data collection was done by M.A. Jiménez-Sousa, L. Rico, E. Largo and E. Golvano.
R. Almansa, J.F. Bermejo-Martín and R. Ortiz de Lejarazu contributed to the writing of the paper. All authors revised the manuscript critically and approved the final version that was submitted.
Competing interestsThe authors declare that they have no competing interests.
We would like to thank to Dr. Lydia Blanco, Head of the “Centro de Hemoterapia y Hemodonación de Castilla y León” who kindly helped us with the logistics of the study. This work was possible thanks to the financial support obtained from the Ministry of Science of Spain and Consejería de Sanidad Junta de Castilla y León-IECSCYL, “Programa para favorecer la incorporación de grupos de investigación en las Instituciones del Sistema Nacional de Salud”, EMER07/050, and “Proyectos en Investigación Sanitaria” PI081236.