The immune system is probably one of the most complex cellular organizations in the body. Its complexity is not superfluous, but rather it is required to fulfill the complicated purpose of the immune system, namely: the recognition of the diverse repertoire of microorganisms and pathogens; the detection of neoplastic lesions originating from a range of tissues; and, while executing these tasks, the maintenance of peripheral tolerance by suppressing detrimental responses against healthy tissues. Since they were discovered by R. Steinman et al. nearly 40 years ago, dendritic cells (DCs) have emerged to be critical players in conducting the immune response to fulfill these roles. Here, we provide a general view on some aspects of DC immunology, highlighting the crucial role that R. Steinman's research in the DC field has played during all those years. This review will also give an outline on DC research in the particular aspects that represent the focus of research groups in Spain (recently organized as the DC.esp working group within SEI). Firstly, some of the subtypes of DC will be described, particularly thymic DC and their role on tolerance; then the DC role in tolerance will be examined, followed by their implications in viral infections. Finally, antigen targeting DCs will be reviewed taking into account the crucial contributions made by R. Steinman et al. This chapter will end by reviewing some DCs based therapies in viral infections.
El sistema inmune es probablemente una de las estructuras más complejas del cuerpo. Esta complejidad no es superflua, sino que es necesaria para realizar todas las complicadas tareas a las que se enfrenta, tales como el reconocimiento de un amplísimo espectro de microorganismos y patógenos, la detección de lesiones oncogénicas en un amplio rango de tejidos y, mientras ejecuta estas tareas, el mantenimiento de la tolerancia periférica mediante la supresión de respuestas perjudiciales dirigidas a tejidos sanos. Desde que fueron descubiertas por R. Steinman y colaboradores hace casi cuarenta años, las células dendríticas (CD) se han posicionado como elementos clave que dirigen el sistema inmune para acometer las tareas propias del mismo. En esta revisión, mostraremos una visión general sobre algunos aspectos de la inmunología de las CD así como subrayaremos el papel primordial que la investigación de R. Steinman ejerció en el campo de las CD durante todos estos años. Además, esta revisión da unas pinceladas sobre la investigación en CD que abordan la mayoría de los grupos de investigación en este campo en España (recientemente organizados en un grupo de trabajo llamado DC.esp, dentro de la SEI). En primer lugar, se describen la mayoría de subtipos de CD, y en concreto las CD tímicas y su papel en tolerancia, para después hablar de la tolerancia y las CD, seguido de los descubrimientos sobre las implicaciones de las CD en infecciones virales. Finalmente, se revisará el direccionamiento de antígenos a CD, teniendo en cuenta las importantes contribuciones de R. Steinman y colaboradores en el campo. Este capítulo finalizará con una revisión sobre las terapias basadas en CD en infecciones virales.
The understanding of the role of dendritic cells (DCs) in immune responses has come a long way since Steinman and colleagues described these cells in 1972. Extensive research during nearly 40 years has provided a good understanding of the complexity of the DC system and its pivotal role in immunity. The following review will attempt to provide a general view on some aspects of DC immunology and to underline the crucial role of Steinman's research on the DC field during all those years. Several groups are working on DC in Spain, some because they were fascinated by DC research and some others because they stumble upon them and they were intrigued about these cells. This review will give an overview on DC research in the aspects that represent the focus of the majority of research groups working in DC immunology in Spain (recently organized as the DC.esp working group within SEI). Firstly, some of the subtypes of DC will be described, particularly thymic DC whose role on tolerance has been recently unraveled and which are in a separated section; then the DC role on tolerance will be examined, followed by a description of DC implications in viral infections. Finally, antigen targeting to DCs will be reviewed taking into account the crucial contributions made by R. Steinman and colleagues. This chapter will end by reviewing some DC-based therapies in viral infections. The role of DCs in cancer and its applications in DC-based cancer immunotherapy are topics not discussed in this review, as they are dealt with by Sureda et al. in other paper in this issue. All authors have contributed similarly in this review and the authorship was decided on alphabetical order.
DCs are integral to the initiation and regulation of immune responses. DCs are professional antigen presenting cells that play essential roles in mediating immunity in front of invading pathogens and also in tolerance to self and innocuous antigens. These apparently opposed functions are performed by DCs due to their plasticity and adaptability to respond to different physiological and pathological stimuli. DCs are located throughout the body and form a refined and complex network that allows them to communicate with different populations of lymphocytes, thereby forming an interface between the external environment and the adaptive immune system. In order to provide this protection, different subsets of DCs have evolved, and these DCs subsets are specialized to exist in distinct locations, where they acquire antigens and transport them to draining lymph nodes for T cell priming. This classification was initially based on their distinct patterns of cell-surface molecule expression. The four major categories of DCs are conventional DCs (cDCs), which predominate in the steady state; plasmacytoid DCs (pDCs); monocyte-derived DCs and Langerhans cells. More information on DCs can be found in Vàquez et al.1
Conventional DCs. Conventional DCs are specialized for antigen processing and presentation. They can be grouped into two main classes based on their localization in tissues and their migratory pathways as they circulate in the body. The first category of conventional DCs is generally referred to as the migratory DCs. Conventional DCs migrate from the blood to peripheral tissues where they sample the environment, and traffic from tissues via apherent lymphatic vessels to local lymph nodes for a highly efficient antigen presentation. Migratory DCs are not found in the spleen and are restricted to the lymph nodes.2 On the contrary, lymphoid tissue-resident DCs do not traffic from other tissues. In the absence of infection, they exist in an immature state (with high endocytic capacity and lower MHC class II expression compared to activated DCs), and their residency in lymphoid tissues makes them ideally placed to sense antigens or pathogens that are transported in the blood.3
Plasmacytoid DCs. pDCs correspond to natural interferon producing cells and are specialized in producing high levels of IFNα in response to virus infection.4 They are quiescent cells that are broadly distributed in the body and they are less competent than cDCs in antigen uptake. They are present in the thymus and secondary lymph nodes but rarely found in non-inflamed tissues and apherent lymphatics. However, under inflammatory conditions, pDCs migrate to and accumulate in tissues and in local lymph nodes, preferentially through high endothelial venules.5
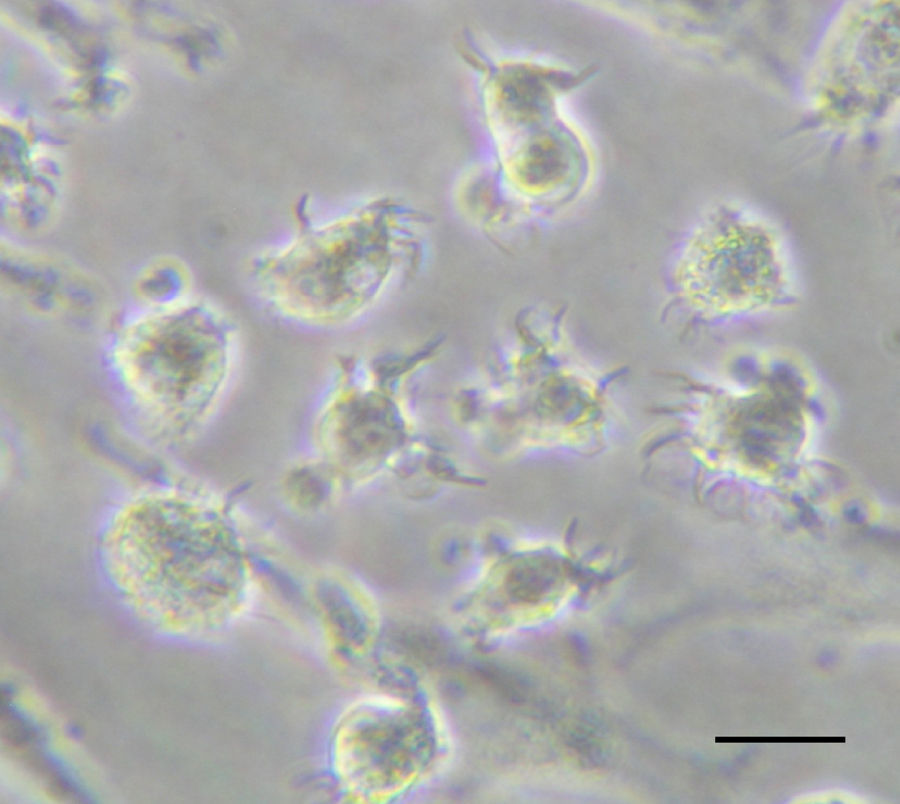
Monocyte-derived DCs. They are induced in response to inflammation when circulating blood monocytes can be rapidly mobilized and can differentiate into cells that possess many prototypical features of DCs (Fig. 1).6 In response to growth factors such as granulocyte–macrophage colony-stimulating factor (GM-CSF) in vitro or to Toll-like receptor 4 (TLR4) ligands or bacteria in vivo, fully differentiated monocyte-derived DCs emerge. In addition, these cells acquire potent antigen-presenting capacity, including the ability to cross-present antigens.7 Thus, it is emerging that monocyte-derived DCs are a crucial reservoir of professional antigen-presenting cells (APCs) that are recruited into immune responses to certain microorganisms and potentially have an emergency back-up role in cases of acute inflammation.
Langerhans cells. Langerhans cells are resident in the skin and, like migratory DCs, migrate to the lymph nodes to present antigens. However, unlike conventional DCs, which arise from a bone marrow precursor cell, Langerhans cells are derived from a local LY6C+ myelomonocytic precursor cell population in the skin. This precursor population originates from macrophages that are present early in embryonic development and that undergo a proliferative burst in the epidermis in the first few days after birth.8
Thymic DCsDendritic cells represent a minor proportion of the hematopoietic compartment present in the postnatal thymus under steady-state conditions. Thymic DCs are able to present antigen in a tolerogenic fashion9 to those CD4+CD8+ double positive (DP) thymocytes that have survived positive selection. Depending on the overall TCR signal quantity and/or quality delivered during this interaction, potentially self-reactive thymocytes are either deleted by apoptosis (negative selection), or undergo commitment towards CD4+ CD25+ Foxp3+ natural regulatory T cells (nTregs), both mechanisms critically contributing to the establishment of central tolerance.10–12 While the role that thymic DCs play in negative selection has been firmly established, their contribution to the generation of nTreg cells remains controversial, especially because thymic epithelial cells (TECs) can function as inducers of nTregs.13–16 Adding complexity to this scheme, several groups have shown that thymus-resident DCs include both pDCs and cDCs subtypes.17–20 In the last years, nTregs have emerged as key players in the induction of immunological tolerance and, therefore, in the protection against fatal autoimmunity throughout life.21 As a consequence, a great interest has emerged in understanding how each intrathymic DC subtype could contribute to the generation of nTregs, especially in humans.
Seminal studies by Liu and coworkers provided direct evidence that human thymic cDCs display tolerogenic potential and generate nTregs, at least under allogeneic conditions in vitro.22 A latter work revealed that only one of the two subsets of cDCs found in mouse thymus supports nTregs development in vivo, and suggested that thymic pDCs display poor tolerogenic function.15 In contrast, we have recently shown that pDCs resident in the human thymus support the differentiation of autologous nTregs from DP thymocytes as efficiently as cDCs, through a mechanism that may involve the CD40-CD40L pathway.23 Moreover, pDCs and cDCs were shown to play a non-redundant functional role, as they selectively induced one of the two nTreg subsets, i.e. IL-10- or TGF-β-producing nTregs, previously identified in the human thymus.24 Therefore, current information points to thymic DCs as key regulators of central tolerance. Whether functional specialization of thymic pDCs and cDCs does exist owing to their distinct antigen presenting abilities25 is an interesting and still open question.
The important tolerogenic role of thymic DCs and their expected therapeutic potential has led to a great interest in defining the developmental origin of these cells. Pioneering work by Ardavin and collaborators showed that early thymic progenitors (ETPs) from the murine thymus displayed cDCs potential in vivo.26 Thereafter, our group extended this finding to human ETPs, which were shown to display non-T cell potentials, including cDCs, macrophage and NK cell potentials in vitro.27–29 As a whole, these data support the notion that thymic DCs are generated in situ from ETPs that display multipotent potential and commit to the T-cell lineage upon thymus entry. More recently, independent studies have shown that human ETPs can also generate pDCs both in vitro30 and in vivo,31 supporting their intrathymic origin. How non-T cells, and specifically DC, developmental pathways branch off from the main T-cell stream has been largely discussed. However, the recent identification in the human postnatal thymus of myeloid-restricted progenitors with pDC/cDC/macrophage potentials (unpublished results) supports a myeloid origin for thymic DCs.
In conclusion, current views support the notion that at least part of thymic pDCs and cDCs are derived in situ from myeloid-primed progenitors. How specific developmental fates are imposed within the thymic microenvironments at specific niches is still an open question that requires further study.
Tolerogenic DCsThe involvement of DCs in central and peripheral tolerance was soon considered as one of the functions played by DCs. It was earlier shown that, under steady-state conditions, iDCs may capture apoptotic bodies derived from natural cell turnover and, after migration to the draining LNs, DCs present self-antigens and silenciate autoreactive T cells.32 It is also well documented that the role of thymic DCs in tolerance induction against self-antigens and numerous studies have revealed mechanisms by which subsets of DCs induce or maintain self-tolerance. Tolerogenic DCs are immature, maturation-resistant or alternatively activated DCs that express low levels surface MHC molecules, have a low ratio of costimulatory to inhibitory signals, and an impaired ability to synthesize Th1- or Th17-cell-driving cytokines (such as IL-12p70, IL-23 or TNF-α) compared with fully mature cells. Furthermore, the expression of inhibitory receptors as well as the secretion of anti-inflammatory cytokines (IL-10 and TGF-β) contributes to tolerance induction. In general, the molecular mechanisms by which DCs exhibit a tolerogenic function include: antigen presentation with inappropriated costimulation (induction of anergy), presentation of very low levels of antigen in the absence of other stimuli, production of cytokines such as IL-10, transforming growth factor (TGF-α), tumor necrosis factor (TNF)-α, granulocyte colony-stimulating factor (G-CSF) or retinoic acid (RA), expression of inhibitory receptors such as ILT3, expression of some molecules that induce T cell death (deletion) such as indoleamine 2,3-dioxygenase (IDO), and also express membrane receptors that may instruct T-cell deletion, such as the interaction through the Fas-ligand, or the programmed death ligands (PDL)-1 and -2. Several evidences support that DCs, through the above mentioned mechanisms, contribute to peripheral tolerance by limiting the expansion of autoreactive T cells and by activating Tregs.33,34
Remarkably the role of DCs in maintaining tolerance is fully independent of their maturation state, since immature DCs, semi-mature DCs and also mature DCs have shown to expand antigen-specific Treg cells both in vitro and in vivo.35–38 Hence, authors recommend defining DCs as ‘immunogenic DCs’ or ‘tolerogenic DCs’ according to their functionality.
Some of the mechanisms indicated above have been exploited for the generation of tolerogenic DCs for therapeutic purposes. Of course, the clinical potential of TolDCs in autoimmunity and transplantation relates in part in the capacity of DCs to induce and expand regulatory T lymphocytes (Tregs) that suppress unwanted immunity. A number of murine studies suggest the possibility that potent antigen-specific regulatory T cells can be readily induced in vivo by targeting antigens to DC specific receptors under non-activating conditions. In certain conditions, mature DCs expand CD4+CD25+ Tregs with retained suppressive activity and are capable of inhibiting diabetes development in NOD mice. Tolerogenic properties of immature DCs have already been documented in healthy human volunteers, providing proof of principle that antigen-specific T cell tolerance can be achieved using this approach in humans.39In vitro, several agents, including dexamethasone, vitamin D3, retinoic acid, or IL-10, can be used to render DCs resistant to maturation when DCs are derived from monocytes (Fig. 1). However, so far, only one recent clinical study has taken advantage of their specific tolerogenic properties by utilizing CD40, CD80 and CD86 antisense transfected DCs to treat diabetic patients.40 The application of these tolerogenic DCs was safe and well tolerated. It is not difficult to speculate that tolerogenic DCs will be evaluated in other autoimmune or chronic inflammatory diseases like multiple sclerosis, arthritis or prevent organ transplant rejection. In vitro experiments have already shown promising results in some of these situations. Moreover, GMP grade TolDCs may be already obtained in GMP facilities, thus further supporting their potential use in a therapeutic scenario.
DCs in viral infectionsA number of viral pathogens can infect cells of the immune system – particularly DCs and macrophages – reducing or even impairing the host's capacity for immune defense. DCs are highly responsive to stimuli such as microbial infection, inflammation, and tissue damage, and their maturation is further driven by interaction with T cells.41 The manner in which different viruses interact with DC function depends both on the virus and on the DC subset involved.42,43 In contrast, the outcome is less dependent on the capacity of the virus to replicate in the DC. A crucial issue is how antigens are presented to T cells by DCs.
Antigen presentation to CD8+ T lymphocytes by virus-infected dendritic cellsA mechanism for initiating CD8+ T-cell immunity to pathogens is the cross-presentation by MHC class I molecules of pathogen antigens after uptake of infected cells by DCs. The most efficient cross-presenting DCs also express the CD8 surface molecule.44 This mechanism is thought to exist to cope with some pathogens that cannot infect immature DCs, such as measles virus,45 or for which infection does not lead to full-blown viral antigen expression in DCs, such as vaccinia virus,46 as well as to deal with some pathogens that compromise the DCs antigen presentation function, such as respiratory syncytial virus,47,48 or that interfere with the MHC class I presentation pathway.49 An extreme interesting case is exemplified by cytomegalovirus, which is cleared by CD8 T-cell immunity in most organs; however, absence of cross-presentation together with interference with direct presentation by MHC class I molecules in the salivary gland result in local virus control exclusively by CD4+ T-cell-produced IFNγ.50
However, for many pathogens, notably for several viruses, direct presentation of viral proteins synthesized in these professional APC upon infection represents a straightforward pathway to prime naïve virus-specific CD8+ T lymphocytes. Interestingly, a need for maturation of influenza-virus-infected DCs results in a delayed direct viral antigen presentation as compared with other cell types,51 while in vivo direct presentation by vaccinia-virus-infected DCs already peaks at 6h post infection.52 The relative in vivo contribution of each mechanism differs for each infection, and there are examples of efficient CD8+ T-lymphocyte priming solely by cross-presentation, such as for herpes simplex virus 1 of for the Ankara strain of vaccinia virus,53,54 or solely by direct presentation, such as for vaccinia virus.55 Actually, it is important to recall that viral antigen presentation by DCs is only relevant if the elicited CD8+ T lymphocytes recognize pathogenically relevant infected tissue cells in the infected organism; for two herpesviruses, cytomegalovirus56 and Epstein-Barr virus,57 DCs have been shown to prime CD8+ T cells specific for viral antigens that are not presented in relevant tissues and that thus do not confer antiviral protection. It is thus very likely that both mechanisms contribute to CD8+ T-lymphocyte-mediated virus control, as the sets of epitopes generated by cross-presentation and direct presentation are not completely overlapping.49
Antigen cross-presentation by dendritic cellsEndocytosed or phagocytosed antigens are generally presented to T lymphocytes as peptide-class II MHC complexes, while endogenous cytosolic antigens are predominantly presented as peptide-class I MHC complexes. Dendritic cells have developed exclusive cross-presentation pathways58 allowing class I MHC-restricted presentation of exogenous antigens, taken up by endocytosis or phagocytosis.59 This pathway allows the induction of immunity or tolerance towards vaccines, pathogens that do not infect DCs, tissue-specific autoantigens, or tumoral antigens. The loading of class I MHC molecules with endocytosed or phagocytosed antigens implies their exit to the cytosol using specific channels to reach the classical class I pathway, or the fusion of the endo/phagolysosomal compartment with the endoplasmic reticulum, where mature class I molecules reside. Although several reports have provided evidences supporting both mechanisms, the routes by which exogenous antigens access newly formed class I MHC molecules remain unclear (revised in60).
The antigen to be cross-presented can be soluble (proteins, lysates) or particulated (pathogens, beads, cell-associated antigens).61 Regarding cell-associated antigen cross-presentation, studies have focused in dead cells (necrotic or apoptotic). In patients with different types of cancer, monocyte-derived DCs (Fig. 1) loaded with autologous or allogenic killed tumor cells have been extensively used for immunotherapy.62 However, only some types of death induce immunogenic stress signals.63 Quite recently it has been demonstrated that human monocyte-derived DCs (Fig. 1) can also acquire antigens from live cells for cross-presentation64 through nibbling, a mechanism similar to trogocytosis.65 It has also been shown that the in vivo cross-presentation of tumoral antigens from live melanoma cells by bone marrow-derived DCs is protective in a therapeutic model.66
In the mouse, CD8α+ DCs were thought to be unique in performing cross-presentation, and their human counterparts (BDCA-3+ cDCs) also present a high cross-presentation activity ex vivo.67,68 However, this cross-presentation activity is not exclusive of the former population and can also be detected in CD1c+ cDCs and pDCs ex vivo.69 Indeed it has also been shown that murine splenic pDCs cells efficiently cross-prime naive T cells in vivo after TLR activation.70 In summary, cross-presentation by DCs is crucial to reach immunoprotection and immunotolerance, but the mechanisms implicated in this exclusive pathway and the DC subsets involved in vivo remain elusive.
Targeting antigens to DCsThe identification of DCs as key regulators of T and B cell adaptive immunity prompted their introduction as adjuvants in vaccination strategies aimed to induce anti-tumor/virus antigen-specific T cell responses. In general, these strategies consist in the isolation of DCs from patients to be then loaded with tumor/viral antigens followed by their in vitro maturation using different stimuli previous to their transfer into the patient. A more direct strategy involves the “in vivo loading” or “targeting” of antigens to DCs through their surface receptors. Ralph Steinman et al. identified and exploited the use of CD205, a C-type lectin receptor (CLR) which is highly expressed in DCs, for the in vivo antigen targeting. CD205 recycles through late endosomal and lysosomal compartments and mediates antigen presentation very efficiently.71 They demonstrated that proteins could be targeted to DEC to greatly enhance antigen presentation to both CD4+ and CD8+ T cells in vivo. They assessed the potential of antigen targeting to dendritic cells to improve immunity, by the incorporation of ovalbumin protein (OVA) into a monoclonal antibody to the DEC-205 receptor. Targeting of anti-DEC-205:OVA to DCs in the steady state induced 4–7 cycles of T cell division, but the T cells were then deleted and the mice became specifically unresponsive to rechallenge with OVA. In contrast, simultaneous delivery of a DC maturation stimulus via CD40, together with anti-DEC-205:OVA, induced strong immunity with the activation of cytolytic CD8+ T cells producing large amounts of interleukin 2 and interferon gamma. They found that DEC-205 provides an efficient receptor-based mechanism for DCs to process proteins for MHC class I presentation in vivo, leading to tolerance in the steady state and immunity after DC maturation.72 Steinman et al. demonstrated that a DEC-targeted vaccine is able to induce a strong and protective CD4+ T cell response, which will improve vaccine efficacy as a stand-alone approach or with other modalities.73 To extend their finding to humans, they coupled the HIV p24 gag protein to anti-DEC antibodies and assessed cross-presentation for recognition by human CD8+ T cells. They found that DCs and DEC-205 can cross-present several different peptides from a single protein, supporting the testing of anti-DEC-205 fusion mAb as a protein-based vaccine for HIV.74,75 When administered together with synthetic double-stranded RNA, polyriboinosinic:polyribocytidylic acid (poly IC) or its analogue poly ICLC as adjuvant, HIV gag-p24 within anti-DEC-205 mAb was found to be highly immunogenic in mice, rhesus macaques, and in ongoing research, healthy human volunteers (reviewed in76). Steinman et al. made a great effort to develop a safe T-cell-based protein vaccine to target antigens to DCs and exploit their pivotal role in initiating adaptive immunity. Many laboratories have extended this approach using a variety of DC receptors. Thus, mannose receptors, DC-SIGN, LOX1, Dectin-1, Fc receptors, CD11c, CD209, CD40 (reviewed in Tacken et al.,77 CD36,78 CD91,79 or different ligands for toll-like receptors such as CpG,80 flagellin81 or the extra domain A from fibronectin82 among others are being exploited to target Ag to DCs and induce humoral and cellular immune responses. Thus, DC-targeted protein vaccines are a potential new vaccine platform, either alone or in combination with other adjuvants, to induce integrated immune responses against microbial or cancer antigens, with improved ease of manufacturing and clinical use.
DC-based therapies in viral infectionsThe properties of DCs as professional APC have led to their use as vaccines in different therapeutic settings. Although most of these applications have been related to the induction of anti-tumor immunity,83 chronic viral infections may also benefit of the advantages of DCs as immunogens. It has been described that among other mechanisms, some viruses may subvert anti-viral immunity by targeting functional properties of DCs,84–87 a feature common to some viruses causing chronic infections. This can be mediated either by a direct effect upon viral infection of DCs or by indirect mechanisms, such as interaction of viral products with DC receptors. Therefore, these individuals are often characterized by their poor T cell responses, mainly those of the Th1 subtype, which display the most potent anti-viral effects.88 The lack of a correct DC function during the natural course of viral infection has suggested that those strategies based on the administration of fully competent DCs may be able to activate T cells with proper anti-viral activity. Thus, initial preclinical studies carried out in animal models showed that DC vaccination could induce potent anti-viral immunity.89 Indeed, it has been shown that in some cases DC vaccination is the most appropriate strategy to break tolerance against viral antigens.90 Regarding human studies, although DCs obtained directly from blood of patients with chronic infections caused by viruses like HIV, HBV or HCV may present some phenotypic and/or functional impairment, protocols have been developed to differentiate functional monocyte-derived from these individuals. In vitro studies using these cells have shown that they can activate T cell responses against viral antigens, suggesting that this approach may be useful in these individuals.91,92 According to it, several clinical trials using DCs have been carried out. HIV infection is the field with the highest number of antiviral therapeutic vaccination clinical trials based on DCs (reviewed in93). They are characterized by their heterogeneity, both on trial design as well as on their DC manufacturing process. Although most of them use monocyte-derived DCs differentiated with GM-CSF and IL-4 (Fig. 1), they include several types of antigens (virus, recombinant proteins, peptides), as well as different loading strategies and maturation stimuli. These clinical trials have shown that DC vaccination is safe in these individuals, showing only minor local side-effects in some patients, in agreement with results obtained in cancer patients. Concerning immunogenicity, DC vaccination induced T cells of the Th1 profile able to recognize viral antigens. However, only in some cases a virological effect was reported, with some one-log decreases of viral load. Other fields using DC vaccination are chronic infections caused by HBV, HCV, HPV and EBV, although few examples exist in these cases.94–97 As for HIV, these protocols have shown to be safe and no significant toxicities were reported. In a similar manner, anti-viral T cell responses were induced or boosted after vaccination. Finally, poor clinical results have been reported, detectable only in a minority of vaccinated patients. The low number of clinical trials as well as their different design and experimental procedures precludes from obtaining solid conclusions regarding their antiviral effect. Nevertheless, current results are promising and further trials are warranted to ascertain the efficacy of DC vaccines as anti-viral therapies.
Future directionsIt is now clear that an extraordinarily complex network of DC subsets contributes in different ways to the induction of immune responses and the maintenance of tolerance. A major challenge now will be to determine the overall contributions of different DC subsets to these processes, to understand the changes in the transcriptional network that are induced by inflammatory states to ensure the mobilization and activation of each DCs precursor and also to decipher DC interactions with other cells in the immune system.
DCs have long been considered ideal candidates for targeted vaccine approaches, but this promise remains largely unfulfilled. However, DC research has reached a point where clinical application has become a real possibility. So far, the rapid pace of immunology research and the fact that a vast number of human diseases have been proven to exhibit an immune or inflammatory component, suggests that the applications envisaged at the moment will likely be expanded in the future in a way that we cannot yet anticipate.
Conflict of interestThe authors declare no conflict of interest.