El encuentro y adquisición de antígeno específico por parte de las células B naïve es el primer estadio de la respuesta inmunológica humoral contra patógenos. Sin embargo, hasta hace poco apenas se sabía cómo, dónde y cuándo las células B naïve encuentran el antígeno in vivo. El desarrollo de modelos experimentales que permiten el seguimiento de antígeno in vivo, junto con la aplicación de tecnología de microscopía confocal y multifotón, han generado datos cruciales y novedosos en el campo. Varios trabajos publicados en el último año muestran el encuentro inicial de antígeno por parte de las células B en ganglios linfáticos drenantes; estos estudios revelan la zona fronteriza entre el folículo y el seno subcapsular (subcapsular sinus, SCS) como un sitio clave donde las células B encuentran antígeno. La capa de macrófagos localizada en la base del SCS tiene una función crítica en la captura, transporte al folículo y presentación del antígeno a las células B foliculares. Además, las células B encuentran el antígeno específico rápidamente tras su administración, lo cual sugiere a las células B como sensores iniciales de la respuesta inmunológica adaptativa. Esta revisión se centra en los últimos datos obtenidos con respecto a los estadios iniciales de la respuesta de las células B.
The encounter and acquisition of specific antigen by naïve B cells is the initial stage of the humoral immune response against pathogens. But, until recently, little was known as to how, where and when naïve B cells find antigen in vivo. The development of experimental models that allow antigen tracking in vivo, in concert with confocal and multiphoton imaging technology, has provided crucial information to the field. Several reports published in the last year show initial antigen encounter by naïve B cells in draining lymph node follicles; all of them point to the follicle boundary with the subcapsular sinus (SCS) as a key site at which B cell antigen recognition occurs. The macrophage sheet at the SCS has a critical function in antigen capture, transport to the follicle and presentation to follicular B cells. In addition, B cell antigen encounter takes place very shortly after antigen administration, suggesting that B cells are initial sensors in the adaptive immune response. This review focuses on the latest data regarding the early stages of the B cell immune response.
B cells are essential effectors of the adaptive immune response to pathogens. They are responsible for pathogen neutralization and clearance by the production of antigen-specific antibodies. The prompt onset of the humoral immune response is thus crucial in the fight against invaders. This process depends mainly on the ability of naїve B cells to hunt for antigen in secondary lymphoid organs (SLO), but also on the efficiency of the mechanisms that drive antigens to the precise site at which they can be encountered. The capacity of the B cell to recognize antigen in its native conformation through the B cell receptor (BCR), in contrast to T cells that requires antigen processing, confers an important advantage that accelerates this process. In this review, recent findings that have provided insights into the mechanisms for antigen delivery directly into the B cell follicles will be discussed, as well as the strategy of naїve B cells for maximizing the efficiency of antigen searching. Finally, novel data derived from imaging studies performed at very early stages of naїve B cell antigen recognition and activation will be commented.
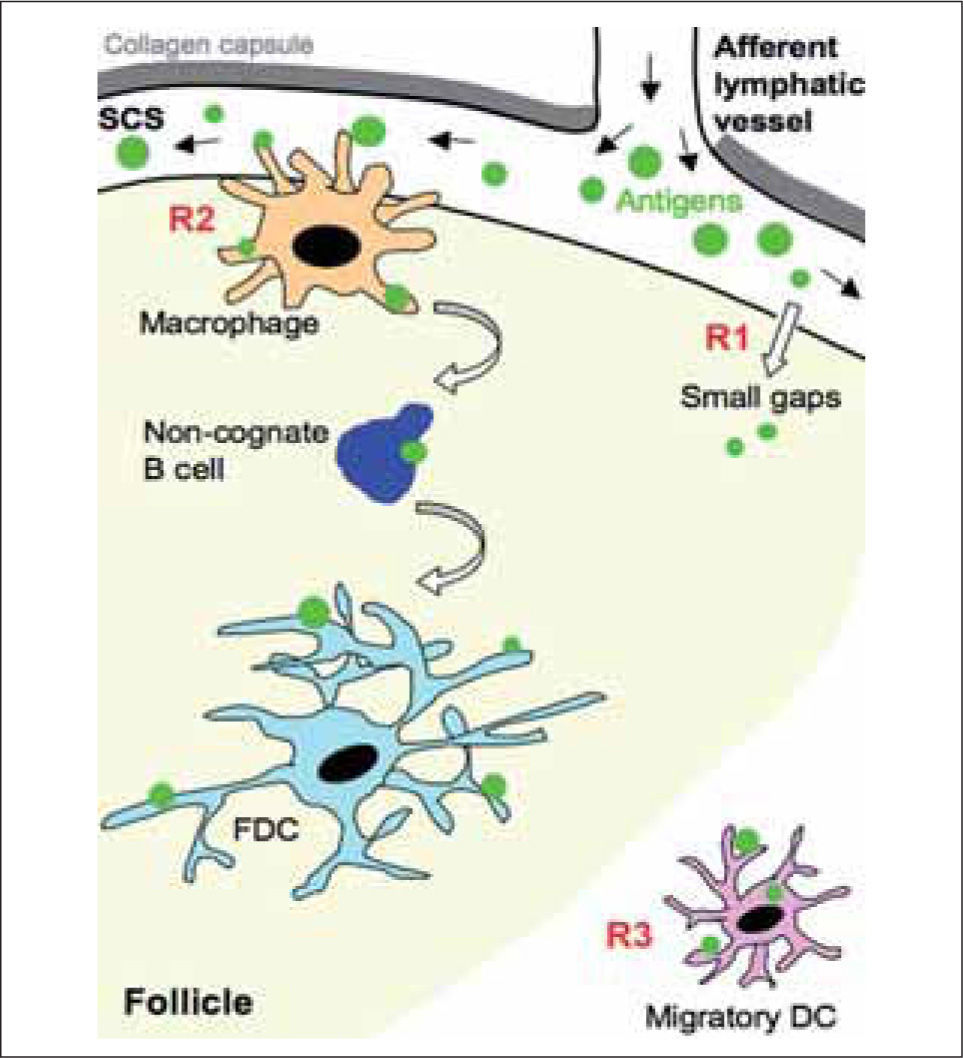
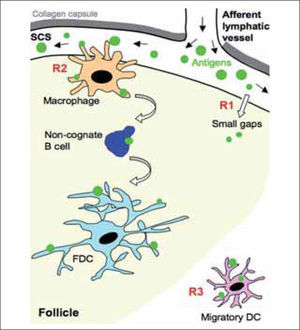
ROUTES OF ANTIGEN DELIVERY INTO FOLLICLESPathogens such as viruses and bacteria enter through peripheral tissues, from which they will be driven to SLO (spleen, lymph nodes, Peyer's patches) by various means. Pathogens and/or antigens can be transported passively from peripheral tissues via lymph to draining lymph nodes, where they drain into the SCS, which separates the collagen capsule from the lymph node cortex(1,2) (Fig. 1). Small antigens are able to filter into the lymph node cortex through the conduit system(1,2), from which they can be captured by resident or accessory cells such as dendritic cells (DC) and follicular dendritic cells (FDC). The conduit system is quite limited in the follicular area, however, suggesting that there are other routes of antigen delivery into the follicle. Moreover, larger antigens such as virus and immune complexes cannot diffuse through the conduit network due to their size(2); they must be actively captured and transported from the SCS into the follicle by accessory or resident cells.
Routes of antigen delivery into draining lymph nodes. Antigens can be transported passively from their entry site at peripheral tissues via lymph into the draining lymph nodes. The afferent lymphatic vessels release them in the subcapsular sinus (SCS); small antigens could diffuse from the SCS into the follicle through the "small gaps" detected in the floor of the SCS by electron microscopy studies (R1). In addition, the resident macrophages located near the SCS capture antigen through the projected protrusions into the lumen of the SCS, and transfer them to the follicle (R2). Follicular naїve B cells take up antigens from the SCS macrophages, carry them to deep locations in the follicle and deliver them to follicular dendritic cells (FDC); this antigen transport by follicular naїve B cells occurs in a BCR-independent, complement-dependent manner. Antigens can also be captured in the peripheral tissue by dendritic cells (Migratory DC) and be transported unprocessed into the draining lymph nodes (R3); migratory DC localize in extrafollicular regions, mainly near the HEV. While the cell-associated form of antigen transport from peripheral tissue into the draining lymph node requires at least 12 h, the passive transport via lymph and uptake by SCS macrophages allows a much faster antigen access to naїve B cells.
Three recent reports from different groups indicate a critical function for a macrophage subset located at the lymph node subcapsular sinus (SCS) in the capture and transport of antigen from the SCS into the follicle(3-5) (Fig. 1 and Table I). Using confocal and multiphoton imaging techniques, they observed that the antigen is retained by these macrophages very shortly (within minutes) after intradermal or subcutaneous administration. Many of these cells project protrusions into the SCS lumen to rapidly capture antigens from the lymph and translocate it into the follicle(4,5). These cells are poorly phagocytic compared to medullary macrophages, and instead retain the antigen on their surface for hours(6). The molecular mechanisms implicated in this process are currently unknown, but may involve different types of receptor, including complement receptors and members of the C-type lectin family(7,8). Depletion of SCS macrophages compromises antigen retention and impairs local B cell activation and response(5). The strategic location of these macrophages is reminiscent of that reported for spleen marginal-zone (MZ) macrophages, which capture blood-borne antigens(9,10). Macrophages thus have an essential role in filtering antigens that arrive from the site of entry via lymph and blood to the SLO, and in presenting them to follicular naїve B cells.
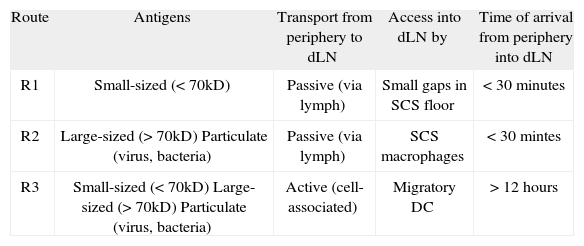
Routes of antigen transport from peripheral tissue into draining lymph nodes. The table summarizes the main characteristics of the distinct routes of antigen delivery from the site of entry at the periphery (skin) into the draining lymph node (dLN). The three routes are also depicted in Figure 1
| Route | Antigens | Transport from periphery to dLN | Access into dLN by | Time of arrival from periphery into dLN |
| R1 | Small-sized (<70kD) | Passive (via lymph) | Small gaps in SCS floor | < 30 minutes |
| R2 | Large-sized (>70kD) Particulate (virus, bacteria) | Passive (via lymph) | SCS macrophages | < 30 mintes |
| R3 | Small-sized (<70kD) Large-sized (>70kD) Particulate (virus, bacteria) | Active (cell-associated) | Migratory DC | > 12 hours |
With time, antigen is also trapped on the surface of the FDC network in the lymph node follicles; the delivery of antigen to the FDC is mediated by follicular B cells in a BCR-independent, complement-dependent manner(3,4) (Fig. 1). Follicular B cells take up antigen in the form of immune complexes from the SCS macrophage protrusions, carry them through the follicle, and deliver them to FDC(4). Follicular B cells thus act as antigen-transporting cells within the follicle. A similar function was previously suggested for MZ B cells in the spleen(11). In a recent study, Cyster and colleagues show the continuous MZ-follicle shuttling of MZ B cells as an efficient mechanism for antigen capture from blood and delivery to FDC(12). All together, these findings support an important function for non-cognate B cells in antigen transport and redistribution, which would facilitate cognate B cell encounter with specific antigen.
Antigens are also transported actively from peripheral tissues into SLO in a cell-associated form. DC can retain engulfed antigen without processing while they travel from epithelial surfaces to lymphoid organs(13,14) (Figure 1 and Table I); this suggests that DC may present intact antigen to B cells. There is considerable evidence of the role of DC as antigen presenting cells for B cells(13,15-17). In addition, naїve B cell antigen recognition was recently visualized on the surface of DC using intravital multiphoton microscopy in lymph nodes(18). Migratory antigen-loaded DC localize in an extrafollicular region near the high endothelial venules (HEV) of the lymph node; newly-arriving naїve B cells encounter antigen on these DC after exiting HEV and before homing into follicles. In contrast to the short-time for antigen arrival via lymph and delivery by SCS macrophages into follicles, migration of antigen-loaded DC from peripheral tissue to the draining lymph node requires at least 12 h(19). The extrafollicular means of B cell antigen encounter may thus act in supporting the B cell response rather than in its initiation, by facilitating activation of B cells newly-recruited to the lymph node.
Finally, electron microscopy studies have detected small gaps (0.1-1 μm) in the SCS floor(20,21), which were recently proposed as an entry site into the follicle for small antigens(22) (Fig. 1). This still controversial route of antigen entry would allow direct access of antigen to naїve follicular B cells by diffusion.
THE NAЇVE B CELL MIGRATION PATTERN: SEARCHING FOR ANTIGENNaїve B cells survey for specific antigen the SLO, where pathogens and other potential antigens are driven. The strategy of naїve B cells to maximize the efficiency of this search is based on their continuous recirculation between spleen, lymph nodes and other structures that form part of the secondary lymphoid tissue network. Naїve B cells enter lymph nodes from the blood through the HEV, and migrate across the T cell zone to localize in the follicular area, where they can spend up to 24 h before exiting through the efferent lymphatics, return to the blood, and repeat the same process(23,24). This homeostatic migration of naїve B cells is orchestrated by members of the chemokine and chemokine receptor families, in particular CXCL13/CXCR5 and by the lysophospholipid sphingosine-1-phosphate (S1P) and its receptor S1P1.
Once naїve B cells arrive in the follicular area, they move at an average speed of 6 μm/min, as observed in multiphoton microscopy studies(25,26). Follicular B cell basal motility depends on chemokine receptor signalling(27), probably on the CXCL13/CXCR5 pair. This dynamic behaviour allows naїve B cells to explore the entire follicular area, thus increasing their chances to find specific antigen. The FDC network in the follicles serves as a scaffold for follicular B cell movement(28). FDC are the main CXCL13 producers in the lymphoid tissue(23), and express high levels of Fc and complement receptors that they use to capture and display antigen to B cells(29,30). Naїve B cells would thus encounter antigens along the paths on which they move. The synergy between these two mechanisms, the dynamic behaviour of naїve B cells within follicles and antigen retention on the substrate for B cell movement, maximize the efficiency of the naїve B cell search for specific antigen.
After a period of random movement in the follicle, naїve B cells return to circulation to continue the search in other SLO. The egress of follicular B cells is regulated by S1P/S1P1(31). S1P is abundant in blood, in contrast with its low levels in lymphoid tissue. It is suggested that the diminished presence of this ligand in lymphoid tissue allows upregulation of S1P1 receptor levels at the cell membrane(24,32); as a consequence, naїve B cells would sense and respond to S1P, overcome retention signals and exit the lymphoid tissue.
EARLY STEPS IN ANTIGEN RECOGNITION AND ACTIVATION OF NAЇVE B CELLSNaїve B cells organize in follicular structures within the SLO. The follicle has long been considered the main site for B cell antigen encounter, with the FDC network as the surface that displays antigen, in the form of immune complexes(30). Nonetheless, there was no bona fide evidence for the relevance of follicular structures and the FDC network in the initial stages of B cell priming. Moreover, the role of FDC in the B cell response has been intensely debated(33,34). The development and recent application of new imaging technology, in particular confocal microscopy and real-time multiphoton microscopy, has provided crucial mechanistic insights into this topic. Using distinct experimental models to track antigen in vivo (soluble antigen, antigen coated-particles, immune complexes, antigen coated-viruses), four groups underlined the primary follicle as the location for antigen encounter by naїve B cells, stressing the boundary between the follicle and the SCS as the main B cell priming site within the whole follicular area(3-5,22) (Fig. 2).
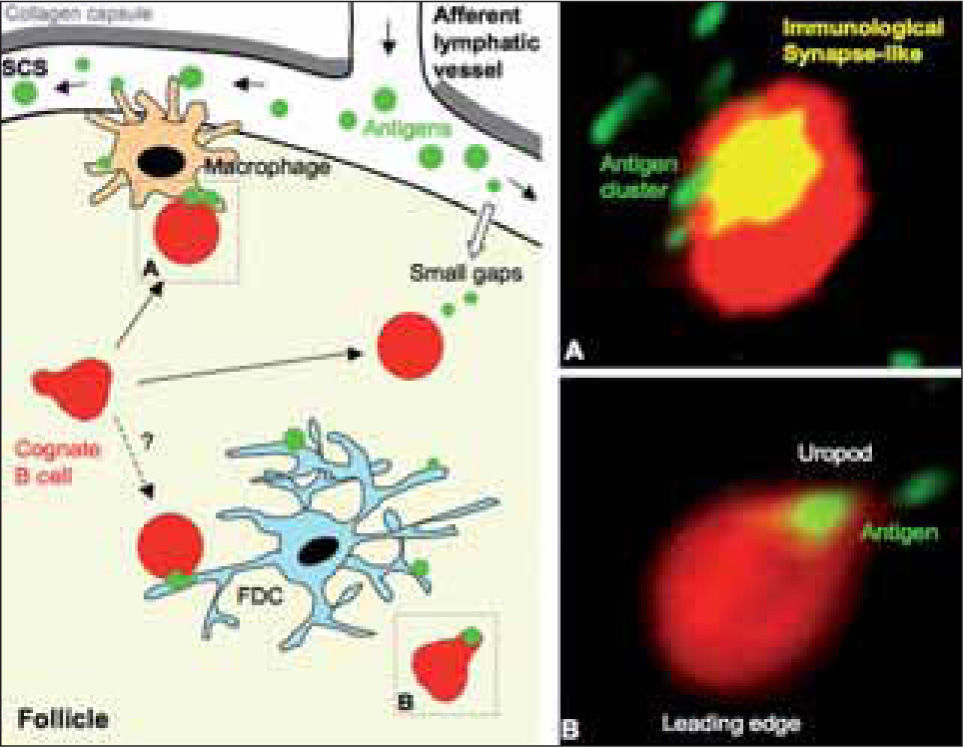
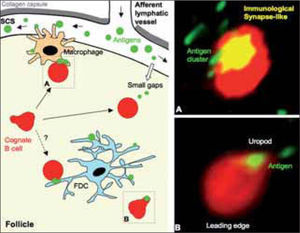
Priming of naїve B cells in draining lymph node follicles. The panel on the left shows a cartoon that summarizes where naїve B cells can encounter specific antigen within the follicle. SCS macrophages have an important role in presenting antigen to naїve B cells, but probably also FDC. In addition, naїve B cells would encounter small antigen that diffuses through the small gaps of the SCS floor. The two different dynamic stages described for antigen-loaded B cells are also highlighted (dashed line boxes) in the cartoon: (A) Naїve B cell of rounded cell shape, mainly stopped, and attached to the antigen-binding site. A cluster of antigen is detected at one side of the B cell (Immunological Synapse-like); (B) Naїve B cell showing a polarized, migratory cell shape, actively migrating and carrying antigen in the uropod. Panels on the right show multiphoton microscopy images of both B cell dynamic stages (A) and (B); naїve B cells and antigen are shown in red and in green, respectively.
The follicles are the main spot for concentration of the diverse repertoire of naїve B cells. The strategic location of follicles close to the antigen-draining zones of SLO (SCS in lymph nodes, MZ in spleen) allows rapid B cell access to incoming antigens. By these two means, concentration and location, naїve B cells ensure efficient detection of cognate antigens.
Tracking antigen recognition in vivoThe tracking of particulate antigen in vivo allows quantitative studies of the initial stages of B cell antigen recognition. By just 60 min after administration of antigen-coated particles, almost 50% of follicular cognate B cells have encountered and captured antigen; frequency increases with time, and by 24 h nearly all cognate B cells in the follicle have encountered antigen, become activated, and migrated to the B cell-T cell boundary, seeking T cell help(3). At very early time points (up to 4 h after antigen administration), antigen-loaded B cells localize only in the upper half of the follicles, indicating that particulate antigen encounter occurs mainly near the SCS(3) (Fig. 2). Moreover, the quantity of particulate antigen per specific B cell increases with time, suggesting that B cells acquire antigen in sequential encounters within the follicle before migrating to the B cell-T cell border(3). The whole process is accelerated when soluble antigen is used(22). The majority of follicular cognate B cells acquire antigen by 10 min after administration; again, initial encounter with soluble or small antigens takes place near the SCS (Fig. 2).
The brief period post-administration required for antigen detection and capture by specific naїve B cells also suggests that B cells resident in the follicle at the time of antigen arrival constitute the majority of the antibody response. In the distinct models analyzed(3-5,22), antigen was administered only once and was consumed mainly by the cognate B cells in the follicle at that time. B cells entering the follicle probably have a role in persistent infections, in which antigen would arrive continuously to the follicle, as well as in later phases of the antibody response.
Although the four reports mentioned above highlight the boundary between the follicle and the SCS as a key site for B cell priming, their observations also suggest additional possibilities. In the absence of large numbers of cognate B cells, antigen is deposited on the FDC surface(3); in addition, non-cognate B cells capture antigen from the SCS macrophages and deliver it to FDC(4). These findings suggest that in normal conditions of B cell clonal abundance, the follicular FDC network may also be a location for naїve B cell antigen encounter (Fig. 2).
In vivo B cell dynamicsMultiphoton microscopy has allowed real time, in situ visualization and study of B cell dynamics during initial stages of antigen encounter in draining lymph node follicles. As discussed above, follicular naїve B cells move by "random walk" at an average velocity of 4-6 μm/min(25,26). Two distinct forms of behaviour are observed relative to naїve B cell localization within the follicle(3). While naїve B cells are highly motile deep in the follicle, they move more slowly and describe short tracks in the zone near the SCS. This may allow careful scanning of the SCS macrophages, crucial route of antigen entry into the draining lymph node. The signal(s) that promote this different behaviour are unknown, which could be due to the distinct substrates of movement (macrophages vs. FDC), to the different chemotactic signals (a chemokine other than CXCL13), or both.
Antigen exposure changes the dynamics of cognate B cells by gradually decreasing their movement within the follicle(3,26). At the earliest time points in the antigen recognition process, antigen-loaded B cells show two distinct forms of behaviour or stages at the single cell level(3) (Fig. 2). In one case, naїve B cells are highly motile, showing a polarized, migratory shape and bearing the antigen in the uropod; these cells are found mainly deep in the follicle. In the second case, the naїve B cells are rounded and are confined to a small area of movement in which they can stay for prolonged periods (>15 min); they appear to be attached to the antigen-binding site, which forms a cluster at one side of the cell. These B cells localize mainly at the boundary between the follicle and the SCS. Antigen recognition thus further reduces B cell movement in the area near the SCS.
B cell immunological synapse in vivoThe rounded B cell shape, its prolonged confinement to a small area with almost no movement, and in particular the formation of an antigen cluster to which it attaches, are all hallmark features of the B cell immunological synapse (IS)(35,36) (Fig. 2). In vitro, this antigen recognition structure is characterized by molecular segregation of receptors into supramolecular activation clusters (SMAC). Initially reported for T cells(37,38) and natural killer (NK) cells(39), B cells also form an IS following membrane-tethered antigen recognition(35,36). IS formation allows B cell triggering under antigen-limiting conditions (low density/low affinity)(36,40), is important for affinity discrimination by B cells, and finally permits more efficient B cell antigen acquisition, essential for later recruitment of T cell help(41). Despite these crucial roles of the IS, in this case for in vitro B cell activation, the relevance and even the existence of the IS in vivo is the subject of animated debate. The multiphoton microscopy findings detailed above suggest the in vivo existence of the B cell IS(3); its relevance for B cell activation in vivo remains to be addressed.
CONCLUDING REMARKSThe recent findings discussed here have shed light on the "how, where and when" of in vivo naїve B cell encounter with antigen. The follicle-SCS boundary in lymph nodes emerges as a key B cell antigen recognition site and the follicles as the location for B cell priming. The SCS macrophages are a device for capturing antigen that arrives via lymph and transferring it to the follicle; they are also important for presenting antigen to naїve B cells. Spleen MZ macrophages may have a similar function, in this case facilitating the presentation of blood-borne antigens to follicular naїve B cells. An important, unexpected role in antigen transport from the SCS macrophages to the follicular FDC network was recently revealed for non-cognate B cells; continuous antigen delivery to the FDC network by non-cognate B cells may facilitate recognition by rare cognate B cells. Finally, the quickness of naїve B cell recognition after antigen administration suggests that B cells are initial sensors and effectors of the adaptive immune response. In conclusion, a more defined picture is now emerging of the initial steps of the B cell response; though the studies discussed here focus their attention in the lymph nodes, it is possible that we will find a similar scenario in other SLO. The development of experimental models that allow real time, in situ antigen tracking and visualization of the early stages of the B cell immune response in vivo has been vital for these advances. It will be exciting to see the data that arise from the use and improvement of these models, for answering the many still-open questions about the nature of the B cell immune response.
DISCLOSURESThe author declare no financial conflicts of interest.
I thank Ignacio Moreno de Alborán and Carlos Ardavín for critical reading of the manuscript, and also Catherine Mark for editorial assistance. Y.R.C. is supported by a Ramón y Cajal contract from the Spanish Ministry of Education and Science. The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.