To characterize a neuron-enriched primary TG culture and evaluate interferon-β expression and activity after HSV-1 infection.
Materials and methodsThe percentage of neurons present in cultures was assessed by neurofilament immunocytochemistry. Cultures were treated with interferon-β and infected with HSV-1, then viral antigen positive cells were counted and interferon-β expression was assessed by quantitative PCR.
ResultsThe culture contained 15% neurons and 85% non-neuronal cells. A cytopathic effect was observed, associated with high viral spread (72.9% neurons and 48.3% non-neuronal cells were positive for viral antigen). Interferon-β treatment impaired the cytopathic effect and decreased the infected neurons to 16.7% and infected non-neuronal cells to 7.8%. Viral infection at 6h post-infection significantly increased the interferon-β transcripts by 18.2 fold, while at 18h post-infection Interferon pre-treatment in infected cultures increased interferon-β transcription by 3.7 fold.
DiscussionThis culture model contained 15% neurons, which is 10 times higher compared to other reported cultures, and non-neuronal cells comprised 85% of cells in this culture. All types of cells were found to be infected, which is similar to that reported during acute infections in vivo. Additionally, interferon-β decreased the infected cells, avoiding the cytopathic effect, which is similar to that reported in swine TG cultures.
ConclusionsA neuron-enriched primary TG model was characterized. Interferon-β treatment protected cells from cytopathic effects and viral spread, while viral infection up-regulated interferon-β expression. This result means that interferon-β exerts an important antiviral effect against HSV-1 in these cultures.
Caracterizar un cultivo primario de ganglio trigeminal (GT) enriquecido en neuronas y evaluar la expresión de interferón-β y su actividad frente a la infección con Herpes simple tipo 1 (HSV-1).
Materiales y métodosEl porcentaje de neuronas fue determinado por inmunocitoquímica para neurofilamento. Los cultivos fueron tratados con interferón-β e infectados con HSV-1, y se cuantificaron las células positivas para antígeno viral por inmunocitoquímica y la expresión de interferón-β por PCR cuantitativa.
ResultadosEl cultivo presentó un 15% de neuronas y 85% de células no neuronales. Se encontró efecto citopático, asociado a una alta diseminación de la infección (72,9% neuronas y 48,3% de células no neuronales positivas para antígeno viral). El interferón-β evitó la aparición de efecto citopático y disminuyó las células infectadas a 16,7% en neuronas y a 7,8% las células no neuronales. La infección viral incrementó la expresión de transcritos de interferón-β 18,2 veces a las 6h de infección, mientras que a las 18h post infección el tratamiento con interferón incrementó esta expresión 3,7 veces.
DiscusiónLos cultivos presentaron un 15% de neuronas, lo cual es 10 veces más que en otros cultivos reportados. Las células no neuronales representan el 85% de las células del cultivo, y se evidenció que todos los tipos de células se infectaron; similar a lo que ha sido reportado durante infecciones agudas in vivo. Adicionalmente, el interferón-β disminuyó el porcentaje de células infectadas y evitó la aparición de efecto citopático, similar a lo que ha sido reportado en cultivos de GT porcino.
ConclusionesSe caracterizó un modelo de cultivo primario de GT enriquecido en neuronas. Interferón-β protegió las células del efecto citopático y la diseminación viral mientras que la infección viral incrementó la expresión de interferón-β. Por lo tanto, el interferón-β ejerció un papel antiviral importante frente al HSV-1 en estos cultivos.
Human Herpes virus 1 or HHV-1, also known as Herpes simplex virus type 1 (HSV-1), is a human pathogen that infects orofacial epithelial cells, leading to cell death and causing vesicular lesions. This stage of the viral replication cycle is called lytic infection.1,2 During this cycle, HSV-1 expresses almost 80 genes that regulate viral replication and virion production. These viral particles are released from infected cells, spreading the infection in the epithelium.1,3 After replication, Herpes virus is transported by axonal transport to infect sensory neurons in the trigeminal ganglia (TG), where it establishes acute infections characterized by viral replication, virion production and further spreading infection in TG cells. This stage is followed by the establishment of latency, characterized by the presence of the viral genome in sensory neurons with a very restricted gene expression profile, without replication or virion production. Once this latent infection is established, the virus remains in the TG for the life of the host, without cure. Under certain stimuli, the virus reactivates, triggering viral gene transcription along with active virion assembly followed by anterograde transport of the virus to the epithelium, causing recurrent epithelial lesions.4–7
To control HSV-1 infections, it has been identified that the immune response mediated by CD8+ lymphocytes and Interferon gamma (IFN-γ) plays an important role in the TG.1 Additionally, it has been reported that endogenous IFN-α protects mice after HSV-1 corneal infections.9 Furthermore, IFN-α/β receptor knockout mice are highly susceptible to HSV-1 infection.10In vitro TG models used to study HSV-1 infection have been characterized by low percentages of neurons, meaning they are composed mainly of fibroblasts.11,15 However, TG sensory neurons are the main infected cell type in vivo, and they are responsible for latency establishment and reactivation.1 Thus, the number of neurons present in the TG model plays a critical role in making it a better approximation to the conditions observed in vivo. It is important to note that in the models previously described, with low content of neurons, the role of IFN-β in TG infected with HSV-1 is not completely understood. For these reasons, this study aimed at establishing a more appropriate in vitro TG model with increased neuron cell proportions, to investigate HSV-1 infection stage in the TG, that could allow a better understanding of the TG response to the infection. To accomplish this, neuron-enriched primary TG cultures were established, the neuronal cells content of this model was characterized by neurofilament immunocytochemistry. In addition, the role of IFN-β against HSV-1 infection in TG was explored in terms of the assessment of changes in viral antigen positive cells and the changes in the expression of IFN-β transcripts, after IFN-β treatment.
Materials and methodsEstablishment of primary trigeminal ganglion culturesAccording to a protocol developed by Castellanos and Hurtado12 using mouse dorsal root ganglia, TG from 4-week-old female ICR mice were dissected. Ganglia were placed in culture media composed of DMEM (Sigma), 10% fetal bovine serum (FBS) (Gibco) and penicillin 100UI/streptomycin 100μg/ml (Gibco). Enzymatic dissociation was performed with 2mg/ml collagenase (Gibco) and 5mg/ml dispase (Sigma) for 1h at 37°C, 5% CO2, and 95% humidity. Mechanical dissociation with a Pasteur pipette was performed every 30min. The dissociated cells were centrifuged at 200×g for 7min, and the cell pellet was resuspended in culture medium with 10% FBS. The cell suspension (50μl per well) was seeded in 24-well culture plates covered with poly-l-lysine treated coverslips (10μg/ml). Afterwards, cell cultures were incubated for 1h at 37°C to allow cell adhesion, and volume was completed with 450μl of culture medium supplemented with 10μM β-D-Arabinofuranoside Cytosine (AraC) (Sigma). TG cultures were incubated for 96h, changing culture media every 24h. At the end of 96h, the medium was replaced with AraC-free culture medium, and cultures were incubated for another 24h before viral infection.
Interferon-β pre-treatment and Herpes Simplex Virus type 1 infection of trigeminal ganglion culturesVirus from a clinical case of herpes labialis was isolated and harvested in the Vero cell line. After 5 days of incubation, cells were freeze-thawed, and supernatants were clarified (10,000×g 10min). Supernatant aliquots were stored at −70°C. HSV-1 in supernatants was then titered by plaque assay in Vero cells (titer was 1×107UFP/ml). HSV-1 was confirmed based on the PCR protocol developed by Kessler et al.13 After a 96h incubation with AraC supplemented media and an 18h incubation in AraC-free culture media, TG cultures were treated with 1000U/ml of murine IFN-β recombinant protein (PBL 12400-1) or culture media alone for 6h before infection. After the incubation period, the medium was washed out, and the cultures were infected with mock (Vero cell lysate) or HSV-1 at a multiplicity of Infection (MOI) of 1, in 5% FBS supplemented culture media. One hour post-infection, the inoculum or mock was removed and replaced with fresh 10% FBS supplemented culture media. Finally, the TG cultures were incubated for additional 28h for immunocytochemistry assays, and for 6, 12 and 18h for quantitative PCR experiments.
Characterization of the trigeminal ganglion cultures with Immunoperoxidase assay for Neurofilament and Herpes Simplex Virus type 1 antigenInfected TG cultures were fixed in 4% paraformaldehyde (Carlo Erba), and monolayers were treated with 0.5% gelatin (Sigma) to prevent cell detachment from the coverslips. Afterwards, monolayers were treated with 0.1% Triton X-100 (Sigma) for 30min and endogenous peroxidases were inactivated with 0.5% hydrogen peroxide in 50% methanol for 45min. Subsequently, blocking was performed with 10% FBS for 30min, followed by incubation either with monoclonal antibody against the specific neuronal intermediate filament (neurofilament) (Sigma A-5316) diluted 1:2000 in 5% SFB for 1h at room temperature, or rabbit anti-HSV-1 (Dako B0114) diluted 1:40 with 5% SFB for 16h at 4°C. Next, biotinylated anti-rabbit (Vector BA-1000) or biotinylated anti-mouse (Vector BA-9200) secondary antibody were added at a 1:200 dilution for 30min at room temperature. Then, 1μg/ml streptavidin-peroxidase (Vector SA-5001) was added and incubated for 30min. Finally, 0.5% hydrogen peroxide with 0.1% Diaminobenzidine (MP Biomedicals) in Tris–HCl pH 7.2 (Sigma) were added for staining. Two independent cultures with three replicas each were evaluated for immunoperoxidase-positive staining.
RNA extraction, reverse transcription and real-time PCR for interferon-β expressionTotal RNA was isolated from TG cultures using Trizol Reagent (Invitrogen) following the manufacturer's instructions. RNA was solubilized in 0.1% v/v Diethyl pyrocarbonate-treated water. Isolated RNA was quantified by A260nm (Nanodrop ND1000, ThermoScientific) and then treated with DNAse I (Sigma), and cDNA was synthesized from 500ng of RNA per sample using random primers and 250U of MMLV (Promega). Relative expression was then quantified using the DyNAmo HS SYBR Green quantitative PCR kit (Finnzymes) in a real-time thermocycler (CFX96 Biorad). The PCR program was as follows: 10min initial denaturation at 95°C followed by 40 cycles of 95°C for 30s and 57°C for 1min for IFN-β and 60°C for 1min for β-actin. Product specificity was verified with a dissociation curve created by reading the fluorescence between 100°C and 60°C. The following primers designed in our group were used: Actin-F: ATC CTC TTC CTC CCT GGA GA, Actin-R: TGC CTG GGT ACA TGG TGG TA, IFNB-F: GCA GCT GAA TGG AAA GAT CA; IFNB-R: TGG CAA AGG CAG TGT AAC TC. β-Actin mRNA was used as a housekeeping normalizer gene. Relative gene expression was quantified by comparing gene expression levels from virus-infected cultures against mock-infected cultures. Relative expression of IFN-β gene was calculated using the mathematical method described by Schefe et al.14 Two independent experiments were performed.
StatisticsSPSS V14 statistical analysis software was used to perform Mann–Whitney test to determine significant differences (p<0.05).
EthicsThis research project was approved by the Universidad El Bosque Ethics’ Committee and it was performed under ethical regulations according to Colombian Health Ministry's Resolution 8430 of 1993. In addition, the research was performed taking into account the Universal declaration of animal rights from the International League of Animal Rights (Law 84 of 1989, Geneva, Switzerland) and according to ethical animal research principles from the International Council for Laboratory Animals.
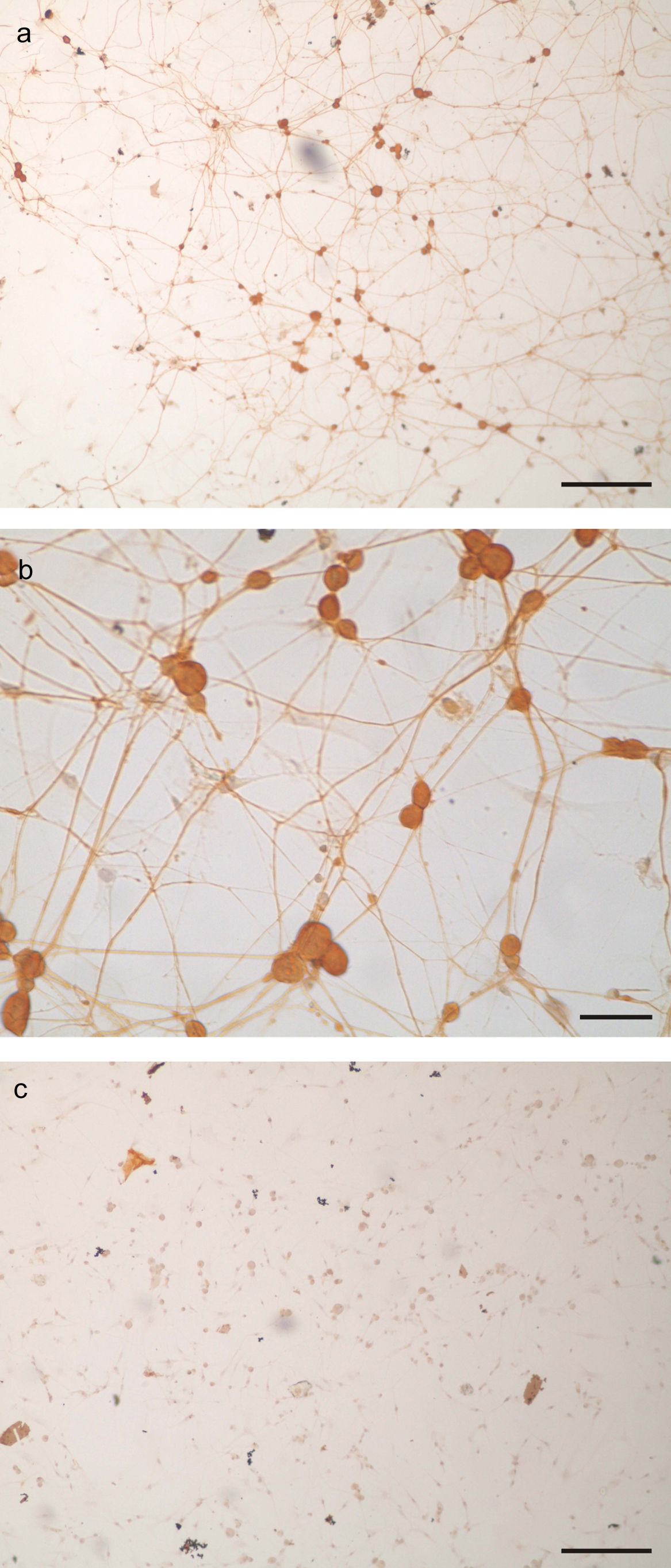
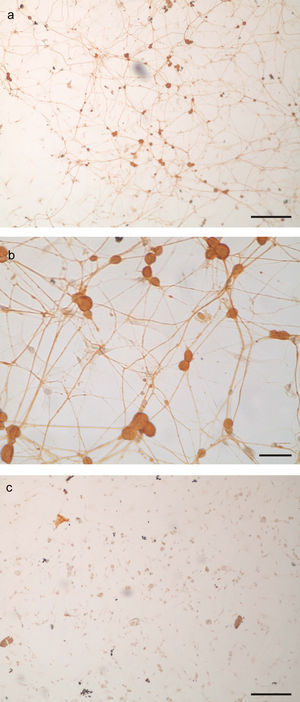
ResultsCharacterization of primary trigeminal ganglion cultures in terms of neuronal and non-neuronal cells contentTG cultures were incubated for 96h with AraC-supplemented medium. The medium was then replaced with fresh AraC-free culture medium, and cultures were incubated for another 24h. Cultures were fixed and stained using an immunoperoxidase assay for neurofilament to characterize their neuronal and non-neuronal cell content. After 120h of incubation, there were 11,000cells per TG culture consisting of 14.7±2.8% of neurons and 85.3±2.8% non-neuronal cells. Thus, a TG culture model was obtained, characterized by the presence of a neurite network that covered the coverslip surface (Fig. 1a and b).
TG primary culture processed by immunocytochemistry for neurofilament protein detection as marker of neuronal cells. a. Panoramic view of neurofilament positive cells (bar represents 200μm). b. Photomicrograph of neurofilament positive neurons. Note the round somas and the immunoreactive neurites (scale bar represents 40μm). c. Aspect of a TG culture processed lacking the neurofilament primary antibody, as negative control. Scale bar represents 200μm.
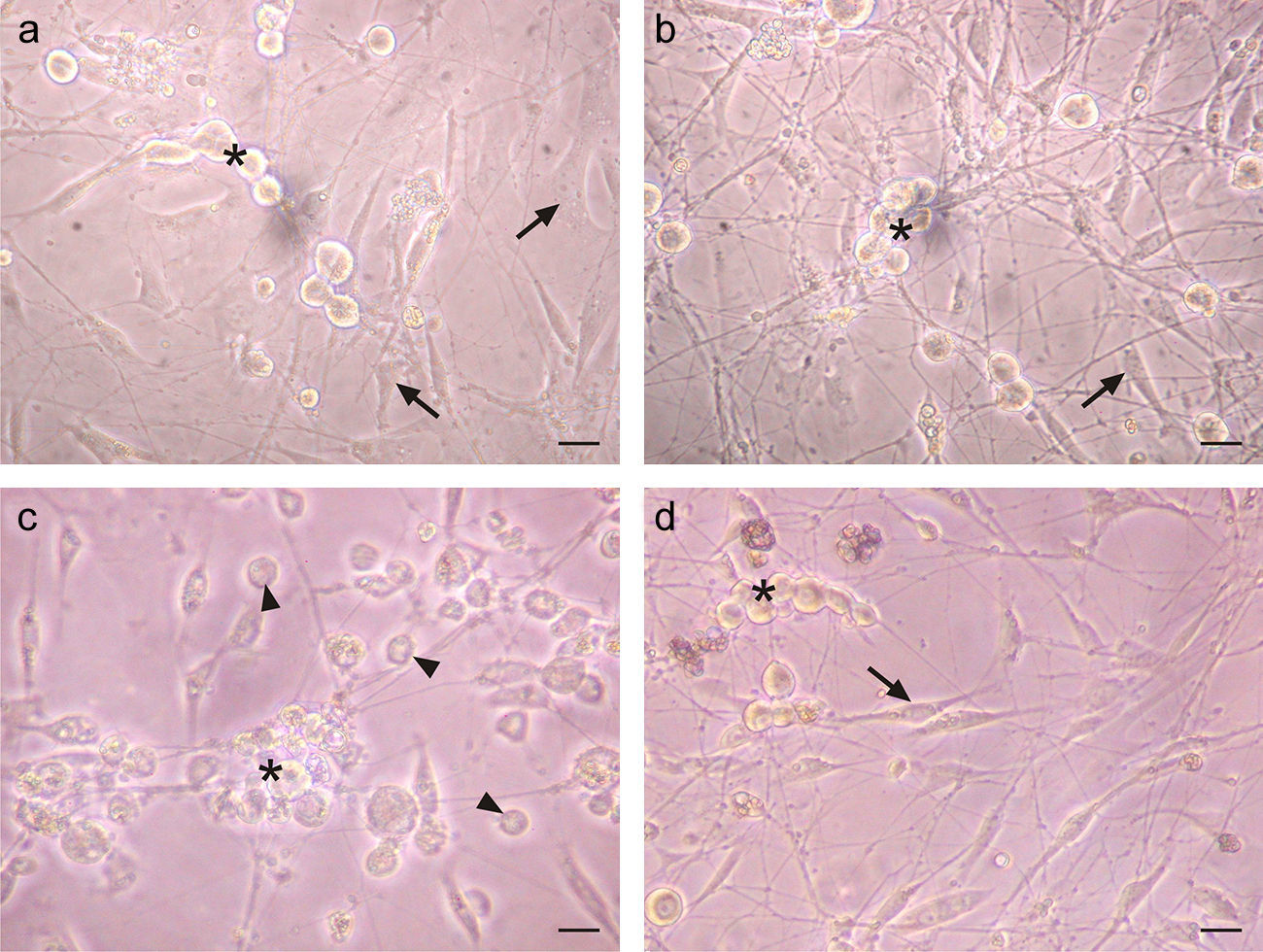
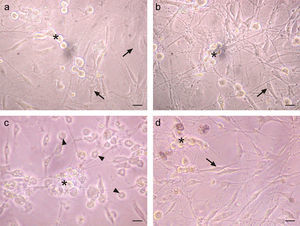
A cytopathic effect was observed in TG cells infected with HSV-1 after 28h. This effect was characterized by the loss of shape, cell rounding and the loss of birefringence in infected non-neuronal cells (Fig. 2c). However, IFN-β pre-treated infected cultures did not show any signs of this cytopathic effect (Fig. 2d), and the cellular morphology of the IFN-β treated infected cultures was similar to uninfected cultures (Fig. 2a and b). This finding suggests that IFN-β impaired the onset of cytopathic effect after HSV-1 infection.
IFN-β protects TG cultured cells from cytopathic effect caused by HSV-1 infection. a. Phase contrast micrographs of TG cultures treated for 6h with 1000U/ml of IFN-β before HSV-1 infection. b. IFN-β untreated and mock infected TG culture. c. IFN-β untreated and HSV-1 infected TG culture. d. TG cultures treated with IFN-β and infected with HSV-1. Note the round and refringent morphology of neurons (*) and the morphology of fibroblasts and Schwann cells (arrows). The virus induced cytopathic effect can be seen in both types of cells (arrow heads). Scale bar represents 40μm. Figure representative of two independent experiments performed.
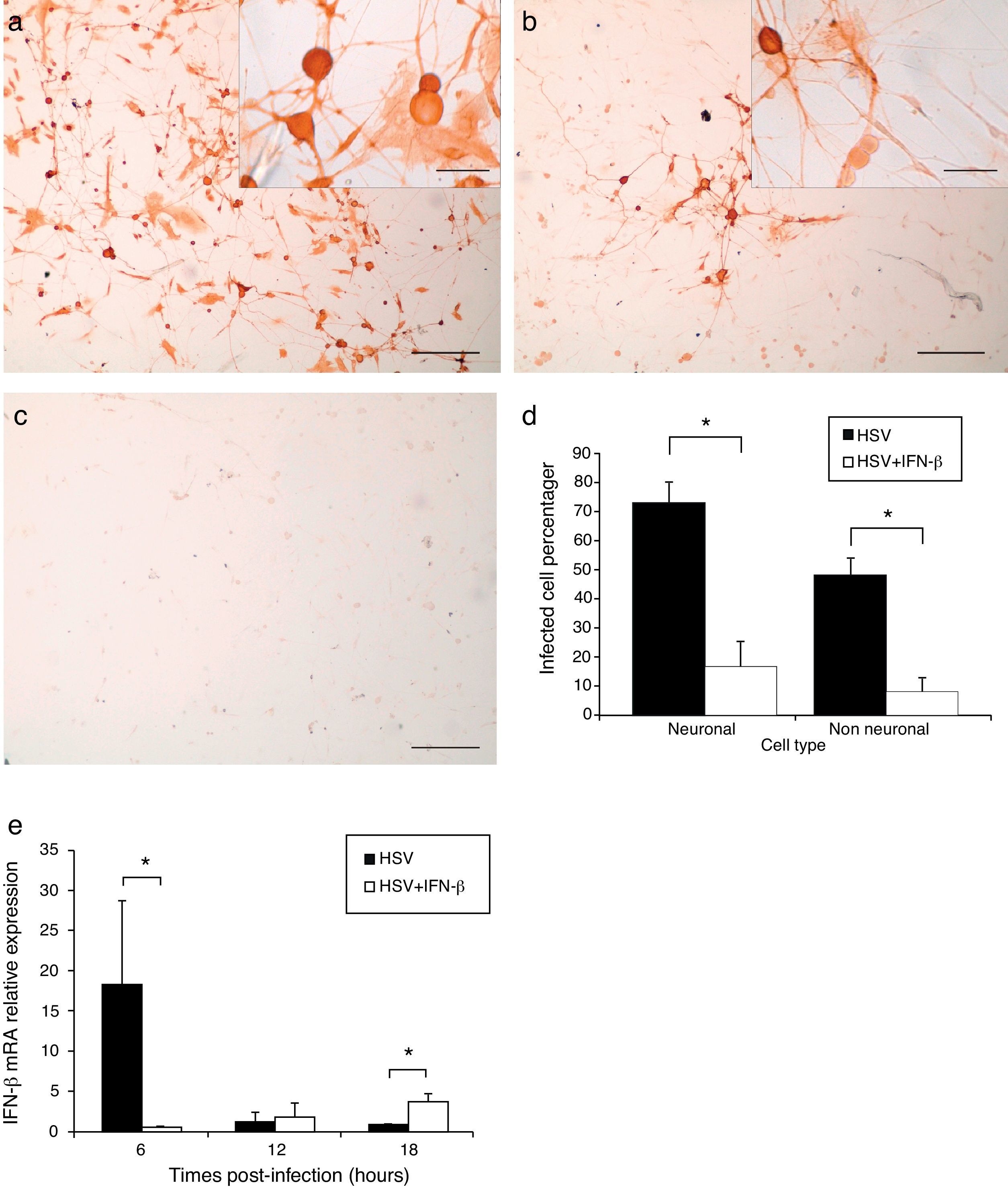
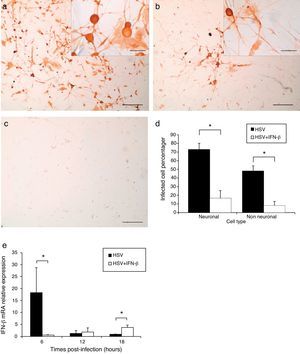
After 28h of infection by HSV-1, the cultures were fixed and processed for immunocytochemistry to detect viral antigen and obtain infected cell percentages. In TG cultures infected for 28h, a more disseminated HSV-1 infection (Fig. 3a) was evidenced, with 72.9% of neuronal cells and 48.3% of non-neuronal cells positive for viral antigen (Fig. 3d). However, pre-treatment with 1000U/ml of IFN-β significantly decreased (Mann–Whitney p<0.05) the number of positive viral antigen cells to 16.7% of neuronal and 7.8% of non-neuronal cells (Fig. 3d). Moreover, IFN-β reduced the viral infection to small isolated groups of infected cells (Fig. 3b). These results indicate that IFN-β treatment had an antiviral effect that decreased the percentages of neuronal and non-neuronal infected cells and impaired virus spread in the TG cultures.
IFN-β treatment impaired viral spreading and decreased infected TG cell percentage. a. Micrograph of immunoperoxidase staining showing viral antigen detection in IFN-β untreated and HSV-1 infected TG cultures. Inset higher magnification of cultures. b. Aspect of IFN-β pre-treated and HSV-1 infected cultures. Note the reduction of virus immunopositive cells. Inset higher magnification of cultures. c. Control culture processed lacking the anti-HSV-1 primary antibody. Scale bar represents 200μm. Inset scale bar represents 40μm d. Viral antigen positive cell percentages in IFN-β treated and untreated cultures. (*) Represents p<0.05 of Mann–Whitney test between HSV-1 infected TG cultures compared to Interferon-β treated and HSV-1 infected cultures e. IFN-β mRNA relative expression in TG cultures infected with HSV-1 for 6, 12 and 18h.p.i. and in cultures pre-treated with IFN-β and infected with HSV-1. Bar represents mean±Standard error of the mean of 2 independent experiments performed by duplicate. (*) Represents p<0.05 of Mann–Whitney test between HSV-1 infected untreated TG cultures compared to HSV-1 infected IFN-β treated TG cultures.
IFN-β mRNA was quantified by quantitative PCR in TG cultures infected for 6, 12 and 18h with HSV-1, and in cultures pre-treated with IFN-β and then infected. Relative expression of IFN-β gene was calculated using the mathematical method described by Schefe et al.14 It was evidenced that HSV-1 infection significantly increased IFN-β transcription in TG cultures by 18.2 fold at 6h.p.i (Mann–Whitney p<0.05), compared to HSV-1 infected cultures. In contrast, in IFN-β pre-treated and infected cultures, IFN-β expression was significantly up-regulated 3.7 fold compared to infected untreated cultures (Mann–Whitney p<0.05). This means that HSV-1 infection triggers IFN-β mRNA expression in early infection of TG primary cultures, and IFN treatment before infection induces IFN-β expression at later stages of infection (Fig. 3e).
DiscussionA neuron enriched primary trigeminal ganglion culture model susceptible to Herpes Simplex Virus type 1 infection was establishedHSV-1 is a neurotropic virus frequently studied in both in vivo and in vitro models. The in vitro model described by Carr et al.11 is a TG primary culture with just 1.5% neuronal cells and a high percentage of non-neuronal cells, mainly fibroblasts.11 Such a low percentage of neurons makes difficult the interpretation of the results, because sensory neurons are the main group of cells infected in vivo and are responsible for latency establishment and reactivations.1 The model described in this work has a higher percentage of sensory neurons, compared to previously reported cultures,11 which could represent a better in vitro approximation of what may occur in TG cells after HSV-1 infection, because the TG sensory neurons are the main cell type infected in vivo, and they are responsible for latency establishment and reactivation.1
In the model described here, the sensory neurons establish a neurite network covering most of the dish surface (Fig. 1a), which most likely increases the interactions between HSV-1 and neurons. In spite of being the smallest percentage of cells in culture, neurons represent the highest percentage of infected cells (72.9%) (Fig. 3d), reflecting the neurotropism of HSV-1. However, in these in vitro circumstances, the entire neuronal cell body and neurite network are available for infection, which could explain this high proportion of neuronal cells infected. This contrasts with the in vivo infection, where virions must enter the sensory nerve endings at the epithelium and travel along the axons to the cell body to establish infections. Evidently, this condition represents a limitation of the model.
On the other hand, the fact that fibroblasts and Schwann cells are the vast majority of cells in the culture, means that they may account for the most part of the response observed, but we cannot establish which type of cell is responsible for the observed results, since all types of cells get infected (Fig. 3a and d). This infection of non-neuronal cells, has also been reported during acute infection periods in mouse models in vivo.15 Therefore, we believe our model is valuable even though the vast majority of cells are non-neuronal type, because here we show that IFN-β induced a response that controlled the viral infection dissemination, impaired the viral induced cell death and decreased the viral antigen expression in TG cells during a productive infection, and it might suggest an important role of non-neuronal cells during acute infections.
Additionally, HSV-1 infection was evidenced at 28h post infection by cytopathic effect (Fig. 2c) and the presence of high number of infected cells (Fig. 3a). This suggests that HSV-1 replicates through a lytic cycle leading to cell death, as previously shown in swine TG culture models infected with HSV-1.16 Moreover, in our model, the highly spread infection observed in all kinds of TG cells, is consistent with the acute infection stage in TG of a mouse model infected in vivo.15 Where HSV positive staining was observed in TG neurons, surrounding satellite cells, as well as in Schwann cells and fibroblasts beginning at 2 days p.i with maximal expression at 3 days p.i.15 These mean that we did not establish a latency model of infection but an in vitro model of acute or productive infection. This could provide information regarding the response of TG cells, neuronal and non-neuronal, against the active herpetic infection that occurs previous to the latency establishment or after reactivation periods, in which the role of non-neuronal cells to control HSV infections remains unclear.
Antiviral effect of interferon against Herpes Simplex Virus type 1 in trigeminal ganglion culturesIFN-β treatment significantly decreased the numbers of neuronal and non-neuronal infected cells. This result implies that TG cells in culture, including neurons, have the ability to respond to exogenous IFN-β stimulation, activating an antiviral state to control HSV-1 infection. This property has been reported in different neuronal cell types such as primary murine cortical neurons, which express antiviral genes such as Protein kinase R after IFN-β treatment,17 and primary murine cerebellar neurons, which increase expression of the antiviral gene Oligoadenylate synthetase after IFN-β treatment.18 On the other hand, in a swine TG culture model infected with HSV-1, the cytopathic effect was impaired, and viral antigen expression was decreased after IFN-β treatment16 and it was also observed in our model. Furthermore, in vivo models have suggested a protective effect of IFN-I (which includes the IFN-α and IFN-β) in acute HSV-1 infections in TG, increasing mouse survival against infection.19–22 Additional studies have confirmed that in vivo HSV-1 replication increases in TG after neutralization of IFN-I with an injection of IFN-I antibody or when the IFN-I receptor gene is deleted in knockout mice.10,23
Moreover, there is evidence suggesting that mouse TG infected with HSV-1 produce IFN-α and -β at 6 days post-infection in vivo,7,8 but there was no evidence that this could also happen in vitro. In our model IFN-β mRNA was up-regulated early in HSV-1 infection of TG cultures (Fig. 3e), which represents the first in vitro TG model in which this was found, because, a previous report of in a TG in vitro model with low percentage of neuronal cells,11 it was found no evidence of IFN production after HSV-1 infection.11 However, in our neuron enriched culture model, we found that the TG cells activate an innate antiviral response against viral infection, which increased IFN-β transcription, suggesting that our model could be a better in vitro approach than previously reported TG cultures. In addition, our group previously reported that after 12h of HSV-1 infection, the IFN induced antiviral genes 2′–5′ oligoadenylate synthetase and latent ribonuclease were up-regulated.24 Since, it has been described that these genes are transcriptionally induced after IFN-β activates its cellular receptor and induces the Jak–Stat signalling,25 we believe that the increased transcription of those antiviral genes, in infected TG cultures, was due to the increased transcription (and possibly increased production) of IFN-β that we found here, triggered by HSV infection. In addition, we evidenced here, that in virus infected and IFN-β treated cultures, a late IFN-β transcription occurred, that we speculate could be explained as a consequence of the autocrine effect induced by the IFN-β treatment, which transcriptionally triggers its own expression.
On the other hand, since the majority of cells in our model are non-neuronal type, it is possible that they are the main responsible for this IFN expression but, at the same time as most neuronal cells are also infected, their possible role as IFN-β producers in this system could not be excluded entirely. The results obtained in this study and those mentioned above, suggest that the IFN-β produced after infection could play an important antiviral role that controls acute HSV-1 infections and prevents virus spread in TG in vivo as well as in vitro.
In conclusion, the novel neuron-enriched TG primary culture model described here was highly susceptible to HSV-1 productive infection that leads to cytopathic effect.
Additionally, we can conclude that IFN-β exerts an important antiviral effect against HSV-1, causing inhibition of the cytopathic effect, decreased viral antigen expression and impaired spread of the infection in monolayers. As a consequence, IFN-β protected both neuronal and non-neuronal cells from HSV-1 infection. But we can conclude that our neuron-enriched culture model could be a better approximation to study HSV acute stages of Herpes infection, compared to other primary TG cultures previously described with low neuron cells percentages. This characteristic makes it a valuable model for further studies that may elucidate the mechanisms behind the acute stages of HSV-1 infection in the TG, previous to latency establishment or even after the reactivation stages. This could lead to a better understanding of the molecular and cellular aspects of TG viral infection. Besides, it could help develop or evaluate putative antiviral treatments, or even establish further in vitro latency models.
Financial supportThis work was financed by Colciencias “Virginia Gutierrez de Pineda” scholarship. Also, it was financed by Division de Investigaciones of Universidad El Bosque and Facultad de Odontología of Universidad Nacional de Colombia.
Conflict of interestsThe authors have no conflict of interests to declare.
Ana Maria Low-Calle was supported by Colciencias Young Researcher “Virginia Gutierrez de Pineda” Scholarship. This work was funded by Division de Investigaciones of Universidad El Bosque and by Facultad de Odontologia, Universidad Nacional de Colombia.