LV intrinsic systolic cardiac function in cirrhotic patients is conditioned by the degree of sympathetic activation and the use of non-selective beta-blockers (NSBBs). Systolic function can be non-invasively measured by ultrasound using Ejection Intraventricular Pressure Differences in the LV (EIVPD). We aimed to address the relationship between systolic function and long-term clinical outcomes using EIVPD.
MethodsWe studied 45 Child–Pugh B or C patients (13 female, 24 on NSBBs) using echocardiography. The primary endpoint was the combination of any-cause mortality or liver transplantation. After a follow-up of 7 years (796 person-months) and a median period of 17 (10–42) months, 41 patients (91%) reached the primary endpoint: 13 (29%) died and 28 (62%) underwent transplantation.
ResultsBy univariable analysis the primary endpoint was related exclusively to MELD score. However, in a multivariable proportional-hazards analysis, adjusted for age, sex and MELD score, EIVPD was inversely related to the primary endpoint, showing interaction with NSBBs. In patients without NSBBs, EIVPD inversely predicted the primary endpoint, whereas in patients with NSBBs, EIVPD was unrelated to outcomes. These relationships were undetected by myocardial strain or conventional cardiac indices.
ConclusionsLV intrinsic systolic function, as noninvasively measured by EIVPD is a predictor of long-term outcomes in patients with cirrhosis. The prognostic value of EIVPD is present along any degree of liver dysfunction but blunted by NSBBs. Because NSBBs have a deep effect on myocardial contractility, these drugs need to be considered when assessing the prognostic implications of cardiac function in these patients.
La función sistólica del ventrículo izquierdo (FSVI) en la cirrosis, está condicionada por la activación simpática y por el uso de betabloqueantes (BBNCS). El gradiente de presión de eyección intraventricular (GPEIV) es un procedimiento ecocardiográfico para la medida precisa de la FSVI. El objetivo del estudio fue evaluar la relación entre la FSVI medida con GPEIV y la evolución de la cirrosis.
MétodosSe estudiaron 45 pacientes Child-Pugh B o C (13 mujeres, 24 con BBNCS) mediante ecocardiograma. La variable principal fue la mortalidad de cualquier causa o el trasplante hepático. Tras un seguimiento de 7 años (796 meses-persona) y una mediana de 17 (10-42) meses, 41 pacientes (91%) alcanzaron el evento primario: 13 (29%) éxitus y 28 (62%) trasplante.
ResultadosEn el análisis univariante, solamente el MELD se asoció al evento principal. En el análisis multivariante de riesgos proporcionales, ajustado por edad, sexo y MELD, el GPEIV presentó una relación inversa con el evento principal, demostrándose una interacción significativa con la toma de BBNCS.
En pacientes sin BBNCS, el GPEIV predijo el evento principal, mientras que en pacientes con BBNCS, el GPEIV no se asoció con la evolución. Esta relación no fue detectada utilizando otros índices convencionales de función sistólica o el “strain” miocárdico.
ConclusiónLa FSVI, medida por el GPEIV se asocia a eventos a largo plazo en la cirrosis avanzada. El valor pronóstico del GPEIV está enmascarado por la toma de BBNCS. Considerando el efecto de los BBNCS en la FSVI, éstos deben ser considerados cuando se analicen las implicaciones pronósticas de la función cardiaca en la cirrosis.
The term cirrhotic cardiomyopathy was coined to describe cardiac disturbances observed in patients with cirrhosis including impaired left ventricular (LV) diastolic function, low cardiac reserve, or arrythmias in presence of normal or enhanced systolic function at rest.1,2 The prognostic implications of cirrhotic cardiomyopathy are controversial.3–6 Some studies have shown a direct relationship between LV systolic and diastolic function and prognosis of cirrhotic patients,6,7 whereas more recent studies have questioned the role of the heart in the pathogenesis of cirrhotic circulatory dysfunction.5,8 However, most studies assessing cardiac function in cirrhosis have relied on indices of global performance, such as LV ejection fraction or cardiac output. These indices, as well as conventional ultrasound-based metrics of diastolic function, are heavily influenced by preload and afterload, and, therefore, particularly unreliable in patients with cirrhosis.5,8–10
The ejection intraventricular pressure difference (EIVPD) is a non-invasive index that accurately accounts for intrinsic global systolic chamber function. The EIVPD is a better surrogate of LV contractility and far less dependent on loading conditions than other metrics, including myocardial strain.11–13 Our group has described that the EIVPD is the most reliable index of systolic function when compared to the gold standard obtained by pressure–volume left and right catheterization in several disease conditions, including patients with decompensated cirrhosis.11,12,14 In patients with cirrhosis, the EIVPD is highly sensitive to the degree of activation of the sympathetic nervous system (SNS), and inversely correlates with the levels of inflammatory plasma biomarkers.12,15 Furthermore, the EIVPD is directly related to the severity of the hepatic disease, patients with more advanced liver disease showing higher levels of SNS activation and higher EIVPD values.12,19 We hypothesized that understanding the relationship between EIVPD and liver outcomes would be useful to clarify the role of cardiac function in the natural history of cirrhosis. Therefore, we performed the first longitudinal study to address the long-term prognostic potential of assessing intrinsic systolic function by means of the EIVPD.
MethodsStudy populationThis study is based on the long-term follow-up data of a previously described, deeply phenotyped cohort of patients with cirrhosis.12 Inclusion criteria were: (1) age between 18 and 70 years, (2) diagnosis of cirrhosis by clinical findings, blood tests, liver ultrasound, transient elastography and/or histology, (3) evaluation for liver transplantation and (4) presence of portal hypertension, defined by characteristic clinical or hemodynamic findings. Exclusion criteria were: (1) any previous history of cardiovascular disease, abnormal regional wall motion assessed by echocardiography or previous history of arrhythmias (or significant electrocardiogram abnormalities, except QT prolongation), (2) significant extrahepatic comorbidities, (3) active infection at the time of the study, (4) history of portal hypertension associated with acute gastrointestinal bleeding in the previous month, (5) portal vein thrombosis, (6) previous insertion of a transjugular intrahepatic portosystemic shunt, (7) Child–Pugh score>13 points, and (8) hepatocellular carcinoma beyond the Milan Criteria. Because the indication for liver transplantation and/or death in all Child–Pugh class-A patients was hepatocarcinoma (a condition that not always reflects the degree of liver failure or portal hypertension), we excluded patients with Child–Pugh class A. Beta-blockers were indicated following clinical guidelines including primary and secondary prophylaxis of variceal bleeding.
The study was approved by the local Ethics Committee and all subjects provided written informed consent for entering the study.
Image acquisition and analysisAll subjects underwent a comprehensive Doppler-echocardiographic exam using broadband 2–4MHz transducers on a Vivid-7 system (GE Healthcare). We measured end-systolic and end-diastolic left ventricular diameters and volumes, fractional shortening, left atrium diameter, peak mitral flow velocities of the early (E) and late (A) filling waves and E-wave deceleration time. Tissue Doppler-derived annular velocities were obtained at the lateral and septal mitral annulus. Circumferential myocardial strain and strain-rate were measured using a commercial software (EchoPac, v.110.1.2, GE Healthcare) from the four and short chamber views, respectively. Remaining echocardiographic indices were obtained following current guidelines.16,17
The EIVPD was measured from Doppler M-mode images of LV outflow obtained from the five-chamber apical view and processed using a custom validated algorithm which numerically integrates blood flow momentum equation.11,12,14,18,19 The EIVPD accounts for the pressure difference between the LV apex and the outflow tract that drives blood flow during systole. The instantaneous peak value of the EIVPD has been demonstrated to be closely related to global intrinsic LV systolic function, a chamber-level surrogate for myocardial contractility.19 Furthermore, this peak-EIVPD has proved to be the most powerful echocardiographic index to characterize LV systolic function in patients with different cardiac conditions undergoing extreme afterload and preload modifications such as those induced by cirrhosis.11,14 The reproducibility of EIVPD has been published elsewhere.19
Clinical follow-up data and the primary endpointBaseline clinical, laboratory, electrocardiographic and imaging data of the original cohort have been previously reported.12 For the purpose of the present study, we underwent an exhaustive review of the electronic medical records of primary care and hospital facilities from all patients up to June 2020. This included access to CIBELES, the administrative database for the Madrid region, which includes death registry of all citizens. Wherever applicable, we recorded the date of liver transplantation, the date and cause of death, as well as the latest contact with the health system for patients without events.
We defined the primary endpoint as a composite of either death (from any cause) or liver transplantation. Liver transplantation was considered as an event because organ substitution changes not only the natural history of the cirrhosis, but also dramatically circulatory conditions and impacts the indications for the use of beta-blockers. Time to the first primary endpoint was recorded for survival analyses, without adjustment for competitive risks.
Statistical analysisQuantitative data are shown as median (interquartile range) except otherwise specified. Non-parametric Wilcoxon tests were used to compare quantitative variables among patients with and without taking beta-blockers. Survival analysis was performed using Kaplan–Meier and proportional hazards models. Multivariable models were pre-defined to include the interaction between EIVPD and use of beta-blockers, given the previously demonstrated effect of the drug on this metric in patients with cirrhosis.12,15 Creatinine was not included in the multivariable model to avoid collinearity with MELD score. Contrast plots of the Hazard Ratios (HRs) along values of EIVPD were calculated for patients on and off beta-blockers. For this purpose, we set the reference value at 3mm Hg, the mean of a normal control population.12 Ninety-five percent confidence intervals (95% CIs) were obtained for all HRs estimations. All statistical analyses were performed in R (version 3.6), expanded by appropriate packages.20 A p<0.05 was considered statistically significant.
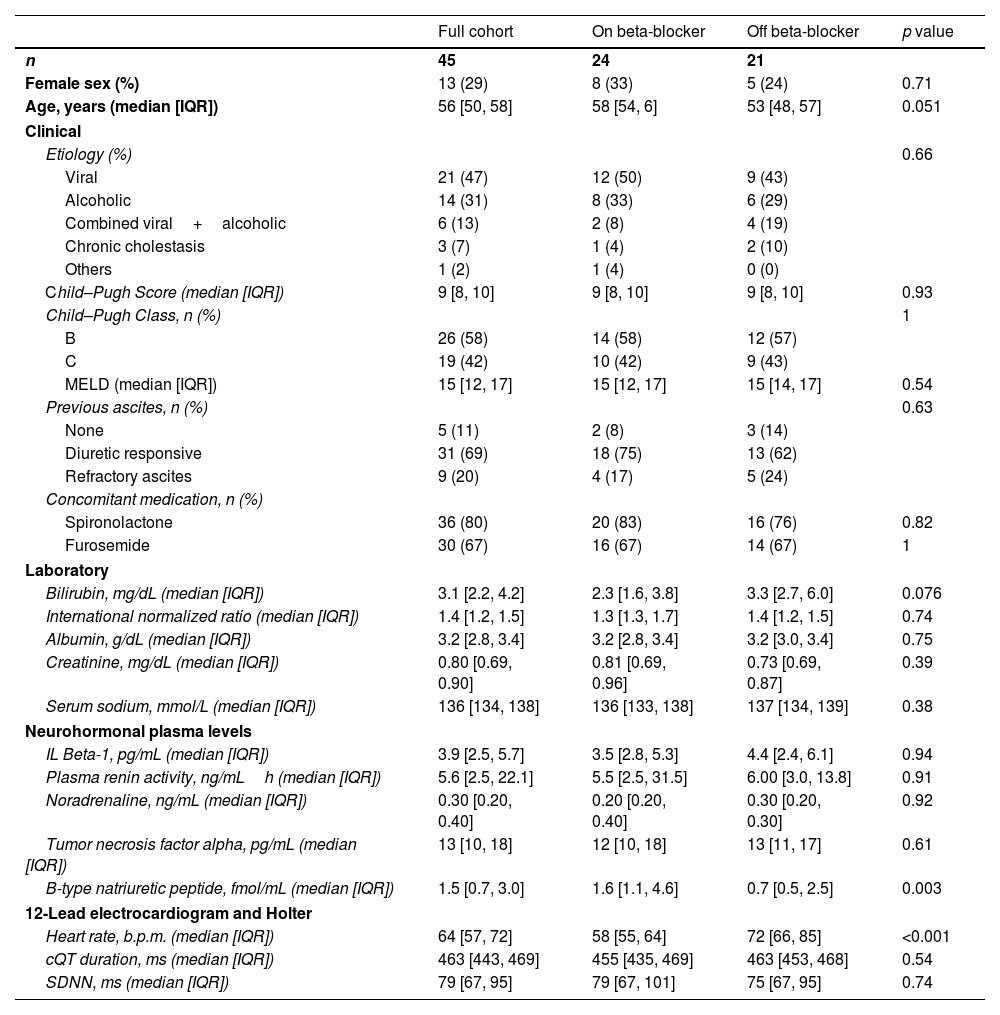
ResultsDemographic and clinical data at enrollment are shown in Table 1. Thirteen of the 45 recruited patients (29%) were women. Median age was 56 (IQR: 50–58) years old. Twenty-four (53%) were on beta-blockers at inclusion. Alcoholic (31%), viral (47%), or a combination of both (13%), were the major etiologies of liver disease. Twenty-six patients (58%) were in Child–Pugh B and 19 (42%) on Child–Pugh C categories at enrollment. Beyond heart rate and plasma levels of BNP (within normal levels) no significant differences were found among patients with or without beta-blockers.
Clinical data at enrollment.
| Full cohort | On beta-blocker | Off beta-blocker | p value | |
|---|---|---|---|---|
| n | 45 | 24 | 21 | |
| Female sex (%) | 13 (29) | 8 (33) | 5 (24) | 0.71 |
| Age, years (median [IQR]) | 56 [50, 58] | 58 [54, 6] | 53 [48, 57] | 0.051 |
| Clinical | ||||
| Etiology (%) | 0.66 | |||
| Viral | 21 (47) | 12 (50) | 9 (43) | |
| Alcoholic | 14 (31) | 8 (33) | 6 (29) | |
| Combined viral+alcoholic | 6 (13) | 2 (8) | 4 (19) | |
| Chronic cholestasis | 3 (7) | 1 (4) | 2 (10) | |
| Others | 1 (2) | 1 (4) | 0 (0) | |
| Child–Pugh Score (median [IQR]) | 9 [8, 10] | 9 [8, 10] | 9 [8, 10] | 0.93 |
| Child–Pugh Class, n (%) | 1 | |||
| B | 26 (58) | 14 (58) | 12 (57) | |
| C | 19 (42) | 10 (42) | 9 (43) | |
| MELD (median [IQR]) | 15 [12, 17] | 15 [12, 17] | 15 [14, 17] | 0.54 |
| Previous ascites, n (%) | 0.63 | |||
| None | 5 (11) | 2 (8) | 3 (14) | |
| Diuretic responsive | 31 (69) | 18 (75) | 13 (62) | |
| Refractory ascites | 9 (20) | 4 (17) | 5 (24) | |
| Concomitant medication, n (%) | ||||
| Spironolactone | 36 (80) | 20 (83) | 16 (76) | 0.82 |
| Furosemide | 30 (67) | 16 (67) | 14 (67) | 1 |
| Laboratory | ||||
| Bilirubin, mg/dL (median [IQR]) | 3.1 [2.2, 4.2] | 2.3 [1.6, 3.8] | 3.3 [2.7, 6.0] | 0.076 |
| International normalized ratio (median [IQR]) | 1.4 [1.2, 1.5] | 1.3 [1.3, 1.7] | 1.4 [1.2, 1.5] | 0.74 |
| Albumin, g/dL (median [IQR]) | 3.2 [2.8, 3.4] | 3.2 [2.8, 3.4] | 3.2 [3.0, 3.4] | 0.75 |
| Creatinine, mg/dL (median [IQR]) | 0.80 [0.69, 0.90] | 0.81 [0.69, 0.96] | 0.73 [0.69, 0.87] | 0.39 |
| Serum sodium, mmol/L (median [IQR]) | 136 [134, 138] | 136 [133, 138] | 137 [134, 139] | 0.38 |
| Neurohormonal plasma levels | ||||
| IL Beta-1, pg/mL (median [IQR]) | 3.9 [2.5, 5.7] | 3.5 [2.8, 5.3] | 4.4 [2.4, 6.1] | 0.94 |
| Plasma renin activity, ng/mLh (median [IQR]) | 5.6 [2.5, 22.1] | 5.5 [2.5, 31.5] | 6.00 [3.0, 13.8] | 0.91 |
| Noradrenaline, ng/mL (median [IQR]) | 0.30 [0.20, 0.40] | 0.20 [0.20, 0.40] | 0.30 [0.20, 0.30] | 0.92 |
| Tumor necrosis factor alpha, pg/mL (median [IQR]) | 13 [10, 18] | 12 [10, 18] | 13 [11, 17] | 0.61 |
| B-type natriuretic peptide, fmol/mL (median [IQR]) | 1.5 [0.7, 3.0] | 1.6 [1.1, 4.6] | 0.7 [0.5, 2.5] | 0.003 |
| 12-Lead electrocardiogram and Holter | ||||
| Heart rate, b.p.m. (median [IQR]) | 64 [57, 72] | 58 [55, 64] | 72 [66, 85] | <0.001 |
| cQT duration, ms (median [IQR]) | 463 [443, 469] | 455 [435, 469] | 463 [453, 468] | 0.54 |
| SDNN, ms (median [IQR]) | 79 [67, 95] | 79 [67, 101] | 75 [67, 95] | 0.74 |
Data presented as median [IQR], except otherwise indicated. SDNN: standard deviation of normal-to-normal RR-intervals.
p values obtained by Wilcoxon test accounts for the comparison between patients with or without beta-blockers.
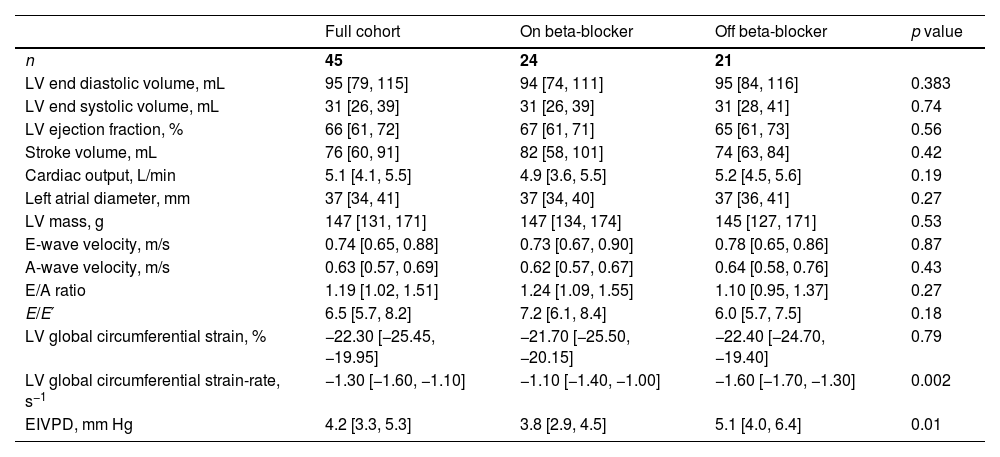
Echocardiographic data at enrollment are shown in Table 2. Conventional indices of systolic function were within normal levels in both groups: cardiac output was 5.1 (4.1–5.5) L/min, stroke volume was 76 (60–91) mL and ejection fraction was 66 (61–72) %. There were no differences between groups regarding indices of diastolic function either. However, EIVPD values and global circumferential strain rate (absolute value) were significantly lower in patients taking beta blockers: [3.8 (2.9–4.5) vs. 5.1 (4.0–6.4) mm Hg; p=0.01] and [−1.10 (−1.40, −1.00) vs. 1.60 (−1.70, −1.30); p=0.002], respectively.
Echocardiographic data at enrolment.
| Full cohort | On beta-blocker | Off beta-blocker | p value | |
|---|---|---|---|---|
| n | 45 | 24 | 21 | |
| LV end diastolic volume, mL | 95 [79, 115] | 94 [74, 111] | 95 [84, 116] | 0.383 |
| LV end systolic volume, mL | 31 [26, 39] | 31 [26, 39] | 31 [28, 41] | 0.74 |
| LV ejection fraction, % | 66 [61, 72] | 67 [61, 71] | 65 [61, 73] | 0.56 |
| Stroke volume, mL | 76 [60, 91] | 82 [58, 101] | 74 [63, 84] | 0.42 |
| Cardiac output, L/min | 5.1 [4.1, 5.5] | 4.9 [3.6, 5.5] | 5.2 [4.5, 5.6] | 0.19 |
| Left atrial diameter, mm | 37 [34, 41] | 37 [34, 40] | 37 [36, 41] | 0.27 |
| LV mass, g | 147 [131, 171] | 147 [134, 174] | 145 [127, 171] | 0.53 |
| E-wave velocity, m/s | 0.74 [0.65, 0.88] | 0.73 [0.67, 0.90] | 0.78 [0.65, 0.86] | 0.87 |
| A-wave velocity, m/s | 0.63 [0.57, 0.69] | 0.62 [0.57, 0.67] | 0.64 [0.58, 0.76] | 0.43 |
| E/A ratio | 1.19 [1.02, 1.51] | 1.24 [1.09, 1.55] | 1.10 [0.95, 1.37] | 0.27 |
| E/E′ | 6.5 [5.7, 8.2] | 7.2 [6.1, 8.4] | 6.0 [5.7, 7.5] | 0.18 |
| LV global circumferential strain, % | −22.30 [−25.45, −19.95] | −21.70 [−25.50, −20.15] | −22.40 [−24.70, −19.40] | 0.79 |
| LV global circumferential strain-rate, s−1 | −1.30 [−1.60, −1.10] | −1.10 [−1.40, −1.00] | −1.60 [−1.70, −1.30] | 0.002 |
| EIVPD, mm Hg | 4.2 [3.3, 5.3] | 3.8 [2.9, 4.5] | 5.1 [4.0, 6.4] | 0.01 |
Data presented as median [IQR], except otherwise indicated. EIVPD: ejection intraventricular pressure difference. p values obtained by Wilcoxon test, accounts for the comparison between patients with or without beta-blockers.
After a median follow-up time of 17 (10–42) months, 41 patients (91%) reached the primary endpoint: 13 patients died and 28 underwent liver transplantation (Fig. 1). This yielded a total of 1796 person-months of follow-up. Causes of death were liver failure in 4 patients, cancer progression before liver transplantation in 3, heart failure in 2, variceal bleeding in 1, and undetermined in 3. One of the patients dying due to heart failure, presented an episode of acute pulmonary edema while undergoing planed vascular surgery 5 years after enrollment; advanced hepatorenal syndrome and moderate LV systolic dysfunction had been diagnosed in the previous 48h before dying. The other patients dying due to heart failure was diagnosed with acute pulmonary edema secondary to acute renal failure and volume overload.
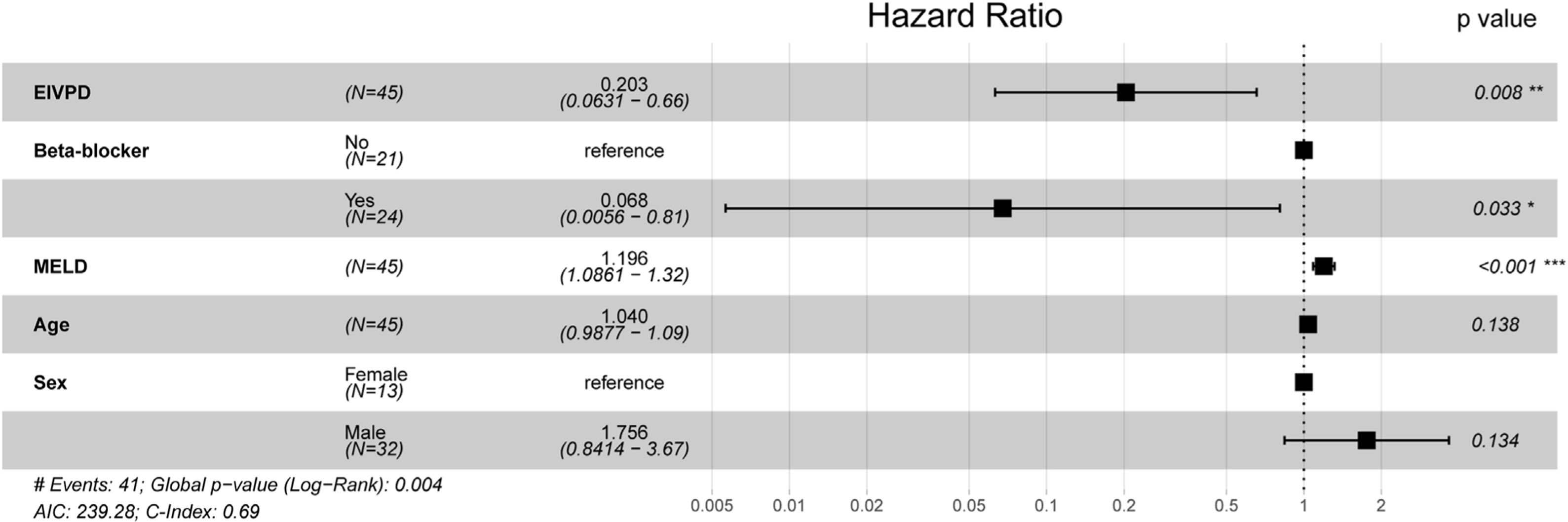
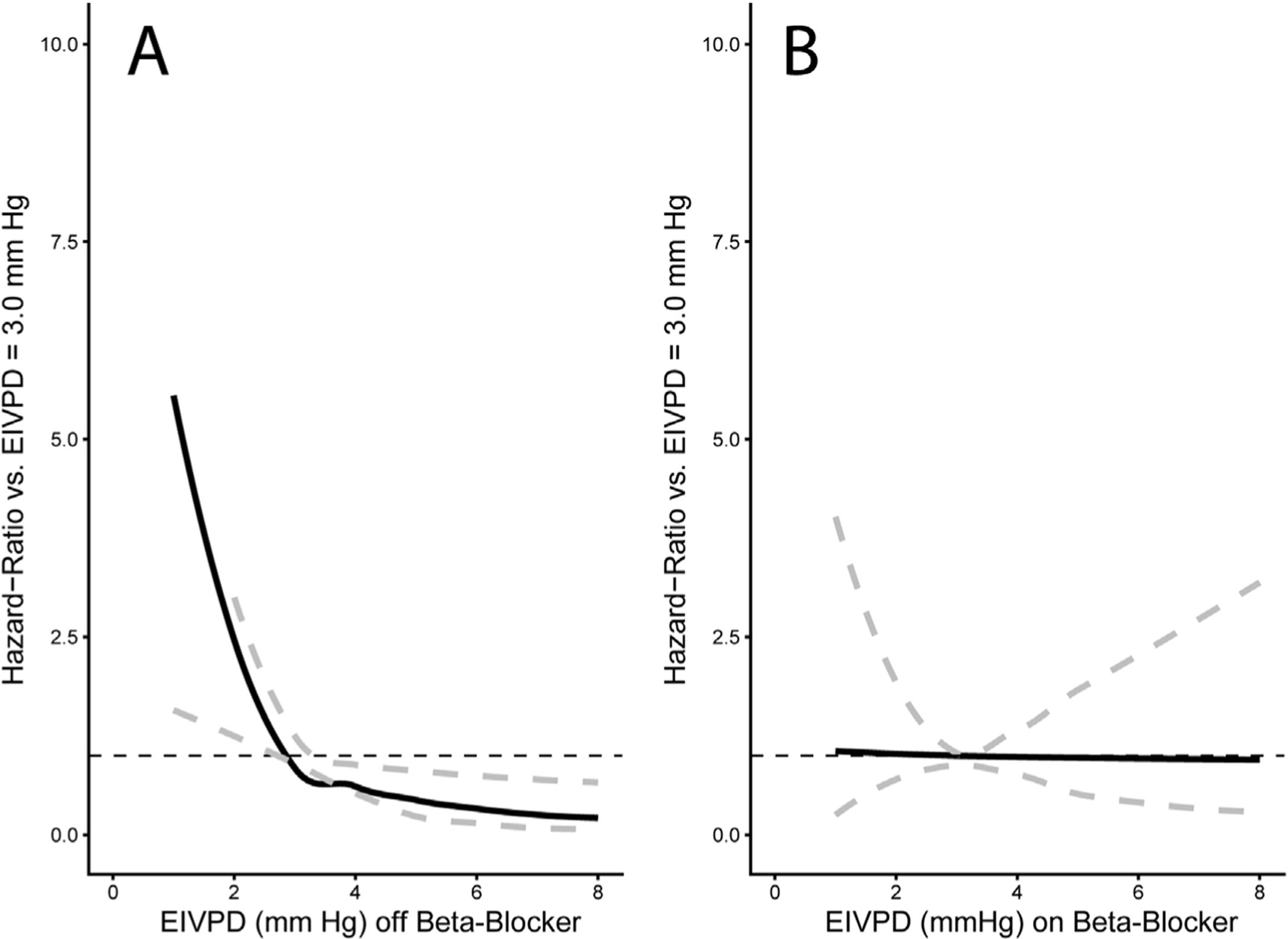
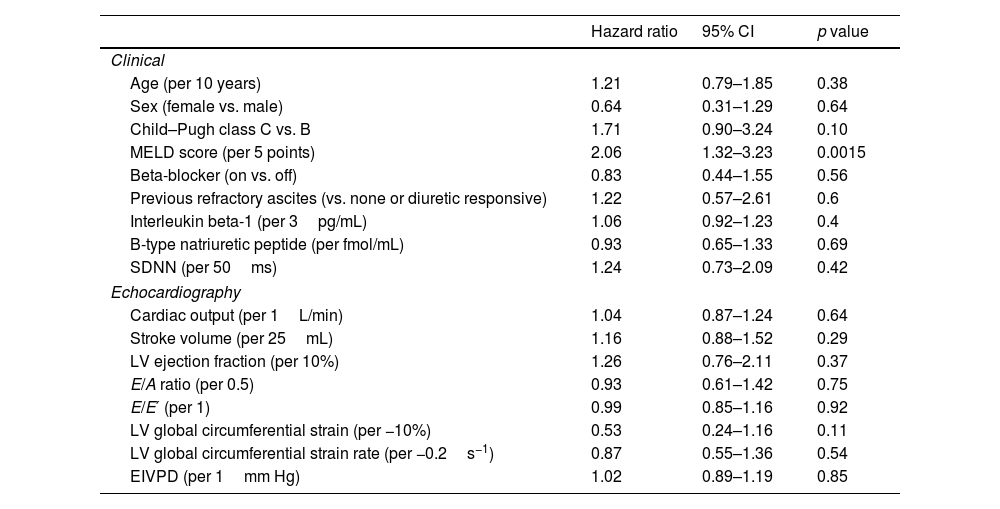
Among clinical and laboratory variables, only the MELD score was related to the primary endpoint in univariable analyses (hazard ratio (HR): 2.06, 95% CI: 1.3–3.2, p=0.001; Table 3). Remarkably, neither the EIVPD (HR: 1.02, 95% CI: 0.89–1.19) nor the use of beta-blockers at enrollment (HR: 0.83, 95% CI: 0.44–1.55) were related to the primary endpoint. However, the multivariable analysis showed interaction between the EIVPD and the use of beta-blockers (p=0.05), which turned significant the effects of EIVPD (p=0.01) and use of beta blockers (p=0.04). The effect of the interaction between beta-blockers and the EIVPD was best demonstrated plotting the HR vs. EIVPD curves for patients off (Fig. 2A) and on betablockers (Fig. 2B). As shown, in patients not taking the drug, there was a strong association between EIVPDs and the primary endpoint: an EIVPD above 3.0mm Hg was protective, whereas an EIVPD below 3.0mm Hg rapidly increased the risk of the primary endpoint (Fig. 2A). In fact, in the subgroup of the n=21 patients not tacking beta-blockers there was a protective effect of a higher EIVPD (MELD-adjusted odds ratio=0.43, 94% CI=0.21–0.89, p=0.02). In patients taking beta-blockers there was no relationship between EIVPD and the primary endpoint (Fig. 2B). Similar data were observed for the sex and age adjusted model (Fig. 3). The prognostic value of EIVPDs were not reproduced neither by LV global circumferential strain nor strain-rate when tested in MELD and/or beta-blocker adjusted models (p>0.1 for all).
Univariate predictors of the primary endpoint.
| Hazard ratio | 95% CI | p value | |
|---|---|---|---|
| Clinical | |||
| Age (per 10 years) | 1.21 | 0.79–1.85 | 0.38 |
| Sex (female vs. male) | 0.64 | 0.31–1.29 | 0.64 |
| Child–Pugh class C vs. B | 1.71 | 0.90–3.24 | 0.10 |
| MELD score (per 5 points) | 2.06 | 1.32–3.23 | 0.0015 |
| Beta-blocker (on vs. off) | 0.83 | 0.44–1.55 | 0.56 |
| Previous refractory ascites (vs. none or diuretic responsive) | 1.22 | 0.57–2.61 | 0.6 |
| Interleukin beta-1 (per 3pg/mL) | 1.06 | 0.92–1.23 | 0.4 |
| B-type natriuretic peptide (per fmol/mL) | 0.93 | 0.65–1.33 | 0.69 |
| SDNN (per 50ms) | 1.24 | 0.73–2.09 | 0.42 |
| Echocardiography | |||
| Cardiac output (per 1L/min) | 1.04 | 0.87–1.24 | 0.64 |
| Stroke volume (per 25mL) | 1.16 | 0.88–1.52 | 0.29 |
| LV ejection fraction (per 10%) | 1.26 | 0.76–2.11 | 0.37 |
| E/A ratio (per 0.5) | 0.93 | 0.61–1.42 | 0.75 |
| E/E′ (per 1) | 0.99 | 0.85–1.16 | 0.92 |
| LV global circumferential strain (per −10%) | 0.53 | 0.24–1.16 | 0.11 |
| LV global circumferential strain rate (per −0.2s−1) | 0.87 | 0.55–1.36 | 0.54 |
| EIVPD (per 1mm Hg) | 1.02 | 0.89–1.19 | 0.85 |
SDNN: standard deviation of normal-to-normal RR-intervals. Other abbreviations as in previous tables.
Interaction plots of the risk of the primary endpoint. The solid line represents the HR along the EIVPD values shown on the horizontal axis whereas dashed gray lines account for its 95% confidence interval. Hazard-ratios express the increase in the risk in the primary endpoint for each EIPVD value compared to the reference value of 3.0mm Hg. Panel A: HR for patients not taking beta-blockers. As shown, in these subjects, the EIVPD has a strong relationship with the primary endpoint: an infra-normal (<3.0mm Hg) EIVPD are an important risk factor for impaired outcomes, whereas a supra-normal (>3.0mm Hg) value is protective. Panel B: HR for patients taking beta-blockers. In this group of patients, there is no association between the EIVPD and the primary endpoint.
Cirrhotic cardiomyopathy is usually subclinical, and its manifestations can emerge in stressful situations5,8 such as exercise,21,22 infections,23 transjugular intrahepatic portosystemic shunt placement24 or liver transplantation.25 Cardiac output decreases during the course of these disease conditions, reaching normal or subnormal values8 and having a negative impact on survival.6,26 However, using appropriate methods for assessing LV systolic chamber function, we have demonstrated that most patients with compensated cirrhosis show enhanced systolic function which correlates with SNS hyperstimulation and the severity of liver disease.12,15,27
EIVPD measurements demonstrate that the enhancement of systolic function observed in patients with cirrhosis using conventional indices (such as ejection fraction) is not only the consequence of the abnormal loading conditions, but also of increased contractility (i.e. the force of muscular contraction).6,11,14 The evidence shown by the EIVPD, has provided useful insight into the cardiac performance and inotropic response of patients with cirrhosis unavailable to other metrics.14,19,28
In two different cross-sectional studies, we have demonstrated that the degree of SNS activation is the strongest determinant of the enhanced systolic LV function found in patients with cirrhosis.12,15 Furthermore, in these two groups we have shown that the hyperstimulation of LV systolic function is modulated by the use of betablockers, and that this drugs has a strong effect on the EIVPD. In the present study, we provide the first evidence on the prognostic potential of these findings. Herein we demonstrate that those patients with advanced cirrhosis who do not show the expected increase in cardiac contractility are at highest risk of unfavorable outcomes. Thus, patients who showed low EIVPD values (typically below<3mm Hg, the average value of controls without cirrhosis) had a higher incidence of the primary endpoint. Importantly, this effect only become apparent in the multivariable analysis adjusted for the severity of liver disease (MELD score). Whether these patients with “lower-than-expected” EIVPD suffer some degree of subtle cardiac dysfunction, impaired autonomous regulation or exhausted overstimulation deserves further investigation. Our data shows that due to their cardiac effects, beta-blockers blunt the prognostic value of LV systolic function as measured by the EIVPD. Interestingly, this interference of beta-blockers with the efficacy of well-validated prognostic biomarkers has been described in other clinical scenarios. In patients with end-stage chronic heart failure, beta-blockers also exert a highly beneficial effect on survival due to their neurohormonal effect. Peak oxygen consumption is a powerful predictor of outcomes in patients with heart failure who are not taking beta-blockers but shows a much more limited prognostic value in patients taking the drug.29,30
The different effect observed in patients receiving beta-blockers is probably due to their global benefits, regardless of their cardiac response. Besides the well-known effect on portal pressure, reducing variceal bleeding and improving survival in patients with and without ascites,31 beta-blockers are also capable of reducing complications such as bacterial translocation32 or hepatorenal syndrome also improving survival in patients with acute-on-chronic liver failure.33,34
The above considerations suggest that sicker patients may benefit most from beta-blockers. However, at this stage, organ perfusion is highly dependent on cardiac output and maintenance of blood pressure.35 Not surprisingly, controversy has risen concerning the possible impact of high doses of beta-blockers on mortality in these clinical settings.36,37 Recently, in a prospective study in patients with refractory ascites we have demonstrated that beta-blockers may induce severe dysregulation and deleterious hemodynamic changes in patients with the highest level of SNS stimulation at baseline.15 Therefore, the use of beta-blockers should be based on a critical risk–benefit assessment in patients with refractory ascites and signs of systemic circulatory dysfunction such as the observed in bacterial infection, bleeding, etc.
The indication of beta-blockers in the cohort was according with well-validated clinical indications. However, although inapparent in terms of clinical and echocardiographic characterization, it is likely that patients not taking beta-blockers were noncomparable to patients taking them in terms of hemodynamic status.
Interestingly, our data did not replicate previous studies7,8 indicating an association between markers of diastolic dysfunction and clinical outcomes. It is important to emphasize that the diagnosis of diastolic dysfunction according ultrasonographic criteria are markedly affected by load conditions, characteristically altered in advanced cirrhosis. Furthermore, the diagnostic accuracy of US measurements as compared with gold standard specific invasive parameters of diastolic function has not been properly validated in patients with cirrhosis.
Study limitationsThe main limitation of the present study is its small sample size. However, the long follow-up period allowed for a high incidence of the primary outcome (91%) and a relatively large number of person-months of follow-up (1796 person-months). Nevertheless, larger ongoing prospective cohorts should confirm the role of measuring LV intrinsic systolic chamber functions using methods as the EIVPD to define clinical outcomes of patients with cirrhosis. Patients were prioritized to receive LT according to the severity of liver disease or hepatocellular carcinoma extension. Thus, the time to the primary endpoint is somehow intrinsically related not only to the degree of liver failure but also to organ availability. Unfortunately, the small sample size precluded more complex statistical analyses (i.e. a pure mortality endpoint and modeling liver transplantation as a time-dependent covariate). Due to the small sample size, any-cause mortality was included in the endpoint. However, non-liver related deaths were only n=3, and therefore had little impact on the observed effects. We believe that entering the MELD score as a covariable in the multivariate analyses should palliate the confounding effect of liver disease severity on the primary endpoint. An additional limitation is the lack of information regarding other prognostic factors.
Clinical implications and conclusionsThese preliminary data suggest that LV intrinsic systolic function, as noninvasively measured by the EIVPD, is a predictor of long-term clinical outcomes in patients with cirrhosis. The prognostic value of EIVPD is independent to the degree of liver dysfunction but blunted by beta-blockers. Because beta-blockers have a deep effect on intrinsic LV systolic function, the effect of these agents need to be considered when assessing the prognostic consequences of cardiac function and dysfunction in patients with cirrhosis.
Sources of fundingSupported by a grant from the Instituto de Salud Carlos IIIPI18/01901. CIBEREHD and CIBERCV are funded by the Instituto de Salud Carlos III with grants co-financed by the European Development Regional Fund (EDRF) “A way to achieve Europe”.
Conflict of interestAll authors: Nothing to disclose.