Autoimmune hepatitis (AIH) is a chronic liver disease of unknown etiology. It can appear after exposure to toxins, being difficult to differentiate it from an immune-mediated DILI (drug-induced liver injury) and a DI-AIH (drug-induced autoimmune hepatitis). In DILI, hypersensitivity symptoms may appear and autoimmune signs may be observed, but the treatment consists in suspending the toxic. In DI-AIH autoimmunity data are observed too but, it is required immunosuppressive treatment, which might be suspended. AIH is characterized by autoimmunity data, a compatible biopsy, and the need to maintain immunosuppressive treatment in the long term. This last point is the main difference with DI-AIH.1
We present the case of a 28-year-old male admitted in our Hospital for severe acute hepatitis. On admission laboratory studies showed GPT 3770U/L, GOT 2276U/L, FA 200U/L, GGT 183U/L, and bilirubin 7.3. Coagulation and a Doppler ultrasound were normal. Hepatopathy screening was performed, everything being normal except for ANA (1/160). IgG levels were 823 but the patient carries HLA DRB1*13, which has been described as a genetic predisposing factor in patients with type I AIH of Caucasoid/North American origin.2 He also carries HLA-B*35:01 which has recently been associated with liver cell damage in individuals taking turmeric.7 He denied the usual consumption of any drug. However, 5 days earlier he consumed cocaine and alcohol. In addition, he reported daily consumption of TURMERIC+(Scientific Nutrition brand). 5 months before admission, at the same time he started the supplementation, analytical alterations were already detectable (GOT 99U/L, GPT 60U/L).
Upon initial suspicion of DILI, turmeric was discontinued. However, liver function parameters worsened. The study was completed with a CT angiography and a liver biopsy. As the study was normal except for autoimmunity, and until the anatomo-pathological result was available, a course of intravenous corticosteroids at a dose of mg/kg was started with subsequent biochemical improvement.
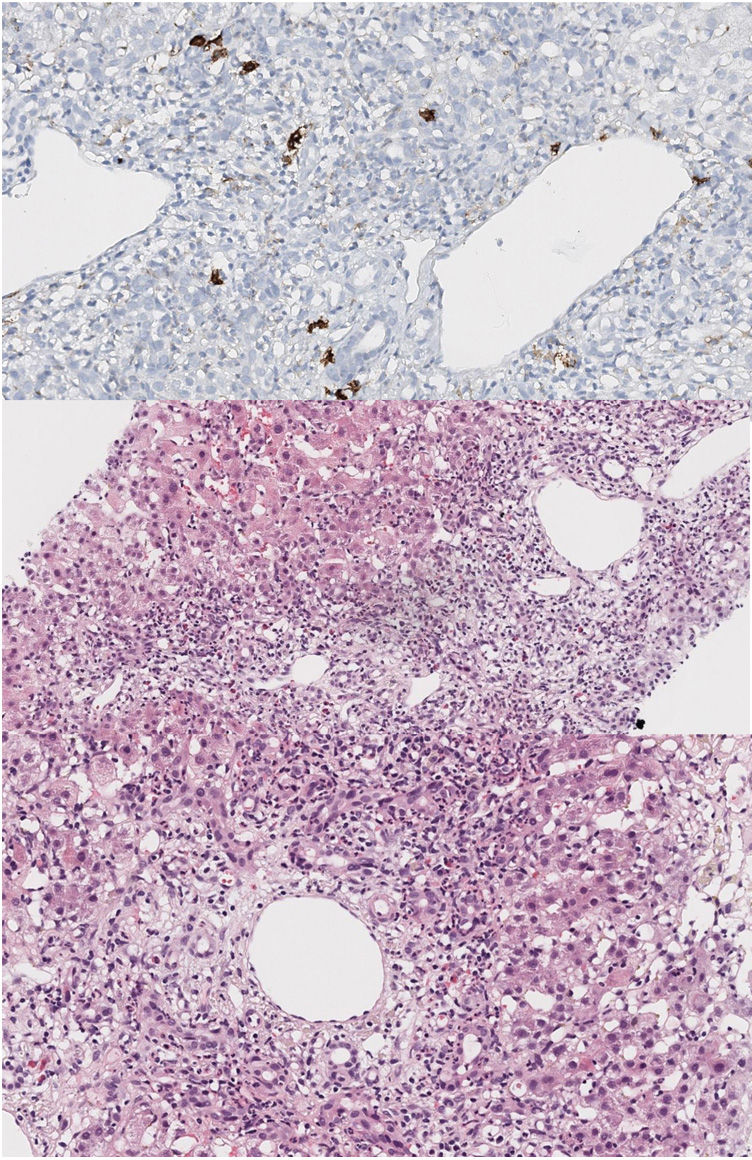
Liver biopsy showed acute portal and periportal inflammation with limiting involvement and a moderate inflammatory cell infiltrate. Lymphocytes, neutrophils and eosinophils were identified, as well as some plasmatic cells (CD38 positive staining). There was piecemeal necrosis and vascular ectasis with discrete sinusoidal dilatation without fibrosis (Fig. 1).
Histological findings. Widening of the portal space with reactive ductular proliferation accompanied by a moderate polymorphous inflammatory infiltrate composed of lymphocytes, neutrophils, eosinophils with presence of some plasma cells (CD38+). The inflammatory infiltrate exceeds the limiting factor, with punch necrosis. Accompanying portal and sinusoidal vascular dilatation.
Azathioprine was associate with analytical improvement. However, a new worsening was observed and forced the increase of the prednisone dose. Nevertheless, it is worth mentioning a doubtful adherence to the treatment. In addition, he suspended treatment and abandoned follow-up for several months. When resumed, transaminase normalization was observed.
Turmeric is a supplement used for antioxidant and anti-inflammatory actions. Its ingredients include turmeric, ginger, Bioperine® black pepper, bulking agent and anti-caking agents among others. Its main component is curcumin. It is often associated with piperine to increase its bioavailability, which could enhance direct toxicity of the turmeric product.3 Turmeric has been implicated causing liver injury, and several cases of liver injury associated with turmeric and curcumin have been published. All of them were resolved after discontinuation of the supplement without receiving immunosuppressive treatment.4–6,8 Ten cases of turmeric-related liver injury have recently been published, highlighting it‘s growing incidence.7
According to the simplified criteria of the International Autoimmune Hepatitis Group, the patient obtained a score of 6 (probable AIH); according to the classic criteria for the diagnosis of AIH, a diagnosis of probable AIH was also reached with a score of 14; furthermore, after calculating the CIOMS-RUCAM score for DILI, a score of 6 (probable DILI) was obtained.
In our case, the histological findings and the predisposing HLA (DRB*13) support the diagnosis of AIH. However, the normalization of liver biochemistry despite the suspension of immunosuppressive treatment and the HLA B*35:01 positivity supports the diagnosis of DI-AIH induced by turmeric intake. Notice that the patient took cocaine and alcohol prior the admission, which may have been a factor influencing the onset of DI-AIH. However, the analytical alterations were noticed long before its consumption.
There is growing evidence that turmeric can induce severe liver injury. Due to this and the analytical normalization after the suspension of immunosuppressive treatment we conclude that the most probable diagnosis for our patient is DI-AIH. However, the number of cases published so far is small, and more, it is needed to establish a definitive causal relationship.
FundingThere was no funding for this article.
Conflicts of interestThere were no conflicts of interest.