In 2022 a subcutaneous formulation of vedolizumab was launched,1,2 leading to the switch from the intravenous to the subcutaneous administration in many patients all over the world.
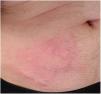
We report a case of a 49-year-old male diagnosed with ulcerative colitis in 1998. In 2004, maintenance therapy with azathioprine was started after a cyclosporine-responding steroid-refractory acute severe flare. In 2015, infliximab was added to azathioprine because of a severe relapse. Five years later, he was treated with intravenous cyclosporine and vedolizumab because of an acute, severe flare, following thereafter vedolizumab in monotherapy. The patient remained in clinical and biological remission and, in September 2022, switching to the subcutaneous formulation of vedolizumab was jointly agreed in accordance to the hospital policy to reduce costs and for patients’ convenience. At the third subcutaneous administration, the patient developed a local skin reaction consisting in a painful oedema and erythema that reappeared in subsequent injections (Fig. 1). For this reason, the patient agreed to return to the intravenous route. At the first intravenous infusion, under the supervision of the Allergology Department, the patient refused the administration of pre-medication and an identical mild skin reaction (erythema, wheals and pruritus) appeared at the site of the last subcutaneous injection (right lower abdominal quadrant) that was administered 4 weeks before. At the time of the subsequent intravenous infusion, the disease was clinically and biologically active, premedication with hydrocortisone (100mg) and intravenous dexchlorpheniramine (5mg) was accepted by the patient and no cutaneous reaction occurred.
The patient remains on intravenous vedolizumab and in clinical remission.
Studies on switching to subcutaneous treatment those patients who are on established maintenance treatment with intravenous vedolizumab are largely lacking. In a recently published series reporting the safety of switching vedolizumab from the intravenous to the subcutaneous formulation in clinical practice, the most frequent adverse event probably related to the treatment was injection site reaction and up to 9% of 82 patients were switched back to the intravenous route due to adverse events or fear to needles.3 In this Dutch multicentre cohort, four out of 135 patients had to switch back to the intravenous one due to injection site reactions. Erythema at the former site injections reappeared in two of these patients, although it did not persist at subsequent infusions (one was pre-medicated with intravenous hydrocortisone and antihistamine).4 Interestingly, Richard et al.5 reported the outcomes of ten patients who switched back to the intravenous route because of injection site reactions with subcutaneous vedolizumab. Among these, seven developed allergic reactions with the intravenous formulation, two of them consisting in erythema at the former injection sites of subcutaneous vedolizumab. The authors suggested that, although the mechanisms involved are unknown, a previous sensitisation during treatment with subcutaneous vedolizumab might be involved.
In our centre, this was the only adverse event noticed among 18 patients after switching to subcutaneous formulation. We believe that reporting such observations may be relevant for clinical practice, particularly if similar cases are reported from different centres, and might advise for caution when considering switching back to the intravenous formulation. For that reason, our recommendation is to discuss the case with the Allergology Department and if the adverse reaction is mild to moderate, consider switching back to the intravenous formulation under appropriate patient monitoring and prior premedication.
FundingNo funding was received for this work.
Authors’ contributionsED prepared the initial manuscript draft, and all authors critically reviewed the manuscript before submission.
Conflicts of interestED has served as a speaker, or has received research or education funding or advisory fees from AbbVie, Adacyte Therapeutics, Biogen, Celltrion, Galapagos, Gilead, GoodGut, Imidomics, Janssen, Kern Pharma, MSD, Pfizer, Roche, Samsung, Takeda, Tillots. FC has served as a speaker for Tillots and received and financial support from Falk Pharma, Otsuka, Ferring. LGG has no conflict of interest to declare.