Pivotal phase studies of telaprevir (TLV) and boceprevir (BOV) showed 10–56% rates of early treatment interruption. However, there have been no reports on the sustained virological response (SVR) rates of these patients.
AimTo assess the SVR rate in a large cohort of patients who discontinued triple therapy with TLV or BOV for reasons other than stopping rules and to identify variables predicting SVR.
Material and methodA survey was sent to 15 hospitals in Catalonia asking them to report all TLV/BOV treatments finished by 31 May 2014. Demographic, clinical, laboratory, liver fibrosis and therapeutic data were recorded for treatments with early discontinuation. Logistic regression analysis, ROC curves and prognostic assessment of the variables identified were calculated.
ResultsTwelve hospitals responded to the survey, representing 467 treatments and 121 (21.2%) early discontinuations, 76 (62.8%) due to stopping rules and 45 (37.2%) for other reasons. Early discontinuation was more frequent with BOV [38.2% (50/131) versus 21.1% (71/336) p<0.005], mainly due to stopping rules [78% (39/50) versus 52.1% (37/71); p=0.004]. SVR was achieved in 21/121 patients (17.4%), 19/71 (26.8%) treated with TLV and 2/50 (4.0%) treated with BOV. In patients discontinuing treatment for reasons other than stopping rules, SVR was achieved in 19/37 (55.9%) treated with TLV and in 2/11 (18.2%) treated with BOV. The SVR rate in patients treated with TLV who discontinued due to a severe adverse event was 61.5% (16/26). A logistic regression analysis was performed only with triple therapy with TLV and early discontinuation. The predictive variables of SVR were undetectable HCV-RNA at treatment week 4 and treatment length longer than 11 weeks. Treatment duration longer than 11 weeks showed the best accuracy (0.794), with a positive predictive value of 0.928.
ConclusionsEarly discontinuation of TLV-based triple therapy due to reasons other than stopping rules still have a significant SVR rate (55.9%). Undetectable HVC-RNA at week 4 of treatment and treatment duration longer than 11 weeks are predictive of SVR in this subset of patients.
Los estudios de registro de telaprevir (TLV) y boceprevir (BOV) han mostrado tasas de interrupción precoz del tratamiento del 10-56%, pero no se ha comunicado la respuesta virológica sostenida (RVS) de estos pacientes.
ObjetivosAnalizar la RVS, y los factores predictivos de esta, en una cohorte extensa de pacientes que pararon precozmente el tratamiento triple con TLV/BOV por causas diferentes a reglas de parada.
Material y métodoSe envió a 15 de hospitales de Cataluña un cuestionario relativo a los tratamientos con TLV/BOV finalizados antes del 31 de mayo de 2014, incluyendo información clínica, analítica, elastométrica y terapéutica de aquellos interrumpidos precozmente. Se realizaron análisis de regresión logística, curvas ROC y estimaciones pronósticas de las variables identificadas.
ResultadosContestaron la encuesta 12 hospitales, sumando un total de 467 tratamientos con 121 (21,2%) interrupciones precoces del mismo, 76 (62,8%) por reglas de parada y 45 (37,2%) por otras causas. Hubo más paradas precoces en los tratamientos con BOV (38,2% [50/131] versus 21,1% [71/336]; p<0,005), principalmente debidas a reglas de parada (78% [39/50] versus 52,1% [37/71]; p=0,004). Alcanzaron RVS 21/121 pacientes (17,4%), 19/71 (26,8%) tratados con TLV y 2/50 (4,0%) tratados con BOV. En los pacientes que pararon el tratamiento por causas distintas a reglas de parada se alcanzó la RVS en 19/37 (55,9%) tratados con TLV y en 2/11 (18,2%) tratados con BOV. Los pacientes tratados con TLV que pararon el tratamiento por efecto adverso grave tuvieron una tasa de RVS del 61,5% (16/26). El análisis de regresión logística se hizo solo con los tratamientos triples con TLV parados precozmente. Las variables predictivas de RVS fueron el ARN-VHC indetectable en semana 4 y la duración del tratamiento mayor de 11 semanas. El mejor valor pronóstico (0,794) lo tuvo la duración total del tratamiento mayor de 11 semanas, con un VPP de 0,928.
ConclusionesLos pacientes que paran precozmente el tratamiento triple con TLV por causas diferentes a reglas de parada conservan una tasa de RVS relevante (55,9%) en esta cohorte. El ARN-VHC indetectable en semana 4 y la duración del tratamiento mayor de 11 semanas son predictivas de RVS de este subgrupo de pacientes.
Telaprevir (TLV) and boceprevir (BOV), first generation protease inhibitors (PI) for the treatment of genotype-1 hepatitis C virus (HCV) infection, were widely used in Spain from early 2012 until late 2014. Their application in the real world setting was complicated by their side effects and by the recommended patient profile (i.e., patients with advanced liver disease).1,2 These two factors may explain the high rate of treatment discontinuation since the introduction of these drugs in association with pegylated-interferon (P) and ribavirin (R). Pivotal phase II and III studies of TLV3–8 and BOV9–11 reported treatment discontinuation rates for any reason between 10.2%4 and 56%.6 Four of these studies of TLV also reported sustained virological response (SVR) rates ranging from 3.8%5 to 50%4 in patients who had discontinued treatment. The ILLUMINATE study8 reported a SVR rate of 23% among patients assigned to the TLV12PR24 and TLV12PR48 study arms who had stopped treatment before week 20. Nevertheless, none of these studies specified the clinical, biological or therapeutic characteristics of the subset of patients who attained SVR. It is reasonable to speculate that patients with SVR and early treatment interruption were the ones who discontinued treatment for reasons other than stopping rules such as side effects, drop-outs or withdrawal of consent.
Recently we reported SVR in 3 patients with HCV RNA which was either undetectable or below 15IU/ml after 4 weeks of treatment, who discontinued TLV-based triple therapy due to severe adverse effects (SAE) at 9, 11 and 12 treatment weeks respectively.12 These patients, members of a cohort of 30 consecutive patients at a single centre receiving triple therapy with TLV, illustrates that SVR may not be an uncommon event after treatment discontinuation for reasons other than stopping rules.
The aim of the study was to assess the frequency of SVR in a large cohort of patients who discontinued early triple therapy with TLV or BOV due to reasons other than stopping rules and to identify variables predicting SVR in these patients.
MethodsStudy designA retrospective study aimed to define the characteristics of a multicentre therapeutic cohort and to identify prognostic variables.
DefinitionsPatients with early treatment discontinuation for reasons other than stopping rules were defined as index cases.
Premature or early treatment discontinuation was considered when the treatment had not reached the scheduled duration on the basis of the fibrosis score (+24) or 48 (4+44) with BOV.
Severe adverse effect (SAE) was defined as an event that entailed risk of death, permanent damage or sequelae or required patient hospitalization for assessment and/or treatment.
Patients and surveyA survey was sent to 15 hospitals in Catalonia asking them to report on the number of treatments with TLV and BOV finished by 31 May 2014 on an intention-to-treat basis, the number of early treatment discontinuations and reasons for interruption. The survey made a special point of recording the reason for the treatment interruption, differentiating those due to stopping rules (i.e., insufficient virological response or loss of response during the treatment) from those occurring while the patient was showing good response to treatment (mainly due to side effects but also due to other reasons such as unmet treatment expectations, unwillingness to comply with treatment and controls, unjustified drop-out, and transient loss of follow-up).
A 19-point questionnaire was administered in index cases, i.e. treatments with premature discontinuation for reasons other than stopping rules. The questionnaire included demographic data (gender and age at the beginning of treatment), laboratory data (platelet count, serum albumin and IL28B haplotype), virological data (subtypes of HCV genotype-1 and HCV-RNA by RT-PCR), liver fibrosis data (kPa and fibrosis score by transient elastography and/or liver biopsy), clinical data (reason for early discontinuation, final outcome of the adverse effect), and therapeutic data (previous treatment experience, PI administered, HCV-RNA at treatment weeks 4 and 12, HCV-RNA 12 and 24 weeks after treatment, duration of PI administration and duration of treatment).
Information was retrospectively collected assuring that all data remained strictly anonymous. The authors processing digital information (DAF and MJFI) did not have access either to the patients included in the survey or the prescription of triple therapy with PI.
To preserve the privacy of the standards of clinical practice at each hospital the survey results were presented in a pooled format.
Statistical analysisEarly discontinuation rates and reasons for discontinuing treatment, whether due to stopping rules of treatment failure or other causes, were calculated from the survey answers. Index cases were analyzed for SVR on the basis of the 19-point questionnaire. Categorical variables were compared by means of the Chi-square test and numerical variables by the Mann–Whitney non-parametric test and presented as median and range. All these tests were 2-sided and p values <0.05 were considered statistically significant. Receiver operating characteristic (ROC) curves of statistically significant numerical variables were obtained and their best cut-off (sensitivity versus 1−specificity) levels used to convert them into categorical variables. All statistically significant variables were studied by logistic regression analysis in order to select the ones with independent predictive value and to calculate their sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy.
Statistical analyses were performed using the SPSS v15.0 software (SPSS Inc, Chicago, IL, USA).
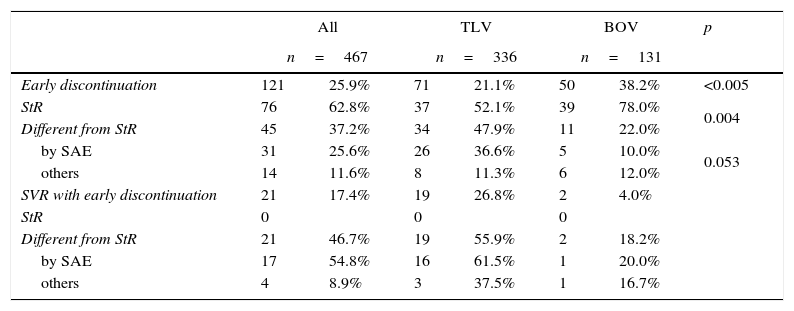
ResultsSurveyTable 1 summarizes the survey results, early discontinuation rates of PI, reasons for early interruption as well as SVR rates in all these categories.
Early discontinuation: results of the survey and SVR rates.
| All | TLV | BOV | p | ||||
|---|---|---|---|---|---|---|---|
| n=467 | n=336 | n=131 | |||||
| Early discontinuation | 121 | 25.9% | 71 | 21.1% | 50 | 38.2% | <0.005 |
| StR | 76 | 62.8% | 37 | 52.1% | 39 | 78.0% | 0.004 |
| Different from StR | 45 | 37.2% | 34 | 47.9% | 11 | 22.0% | |
| by SAE | 31 | 25.6% | 26 | 36.6% | 5 | 10.0% | 0.053 |
| others | 14 | 11.6% | 8 | 11.3% | 6 | 12.0% | |
| SVR with early discontinuation | 21 | 17.4% | 19 | 26.8% | 2 | 4.0% | |
| StR | 0 | 0 | 0 | ||||
| Different from StR | 21 | 46.7% | 19 | 55.9% | 2 | 18.2% | |
| by SAE | 17 | 54.8% | 16 | 61.5% | 1 | 20.0% | |
| others | 4 | 8.9% | 3 | 37.5% | 1 | 16.7% | |
TLV, telaprevir; BOV, boceprevir; StR, stopping rules; SAE, severe adverse effect; SVR, sustained virological response.
Twelve out of 15 hospitals answered the survey, summing a total of 467 triple therapies (hospital treatment range 5–94), 336 with TLV and 131 with BOV. All the hospitals had TLV-treated patients but only 7 had prescribed BOV.
Early treatment discontinuations were reported in 121 patients (25.9%), 76 (62.8%) due to stopping rules of treatment failure and 45 (37.2%) for other reasons. Among this latter group, 31 discontinuations were due to SAE (25.6%) and 14 (11.6%) to general malaise, unmet treatment expectations, unwillingness to continue complying with treatment and controls, unjustified drop-out or transient loss of follow-up. No treatment was resumed once interrupted.
TLV and BOV showed different patterns in terms of reasons for discontinuation. Treatments with BOV were associated with more early discontinuations (38.2% versus 21.1%; p<0.005) and manly due to stopping rules (78% versus 52.1%; p=0.004). Regarding discontinuation for reasons other than stopping rules, a trend was found towards a higher prevalence of SAE among patients treated with TLV (p=0.053). Median time-to-discontinuation in index cases was 9.5 (0.5–36) weeks in TLV- and 14 (5.0–36) weeks in BOV-treated patients (p=0.026).
SVR was attained in 21 out of 121 patients (17.4%) with early treatment discontinuation, in 19 out of 71 (26.8%) of those treated with TLV and in 2 out of 50 (4.0%) treated with BOV. In patients who interrupted treatment for reasons other than stopping rules, SVR was attained in 19 out of 37 (55.9%) treated with TLV and in 2 out of 11 (18.2%) treated with BOV. TLV-treated patients who discontinued the treatment because of SAE showed the highest SVR rate (61.5%).
The shortest treatment resulting in SVR in the 34 TLV-treated index cases was 4 weeks in a F4, IL28B CC, naïve patient who discontinued due to a severe and unexpected psychotic outbreak. Three other TLV-treated patients attained SVR after 6 weeks of treatment (2 relapsers, 1 naïve, all were F4 and interrupted due to SAE). Only 2 out of 11 BOV-treated index cases attained SVR: 1 after 24 weeks of treatment (a relapser, F3, with undetectable RNA at week 8 and a scheduled treatment duration of 28 weeks) and the other after 29 weeks (another relapser, F4, with undetectable RNA at week 8 and a scheduled treatment duration of 48 weeks) of treatment.
All patients with SAE recovered uneventfully although 1 patient required anti-depressive therapy for 1 year.
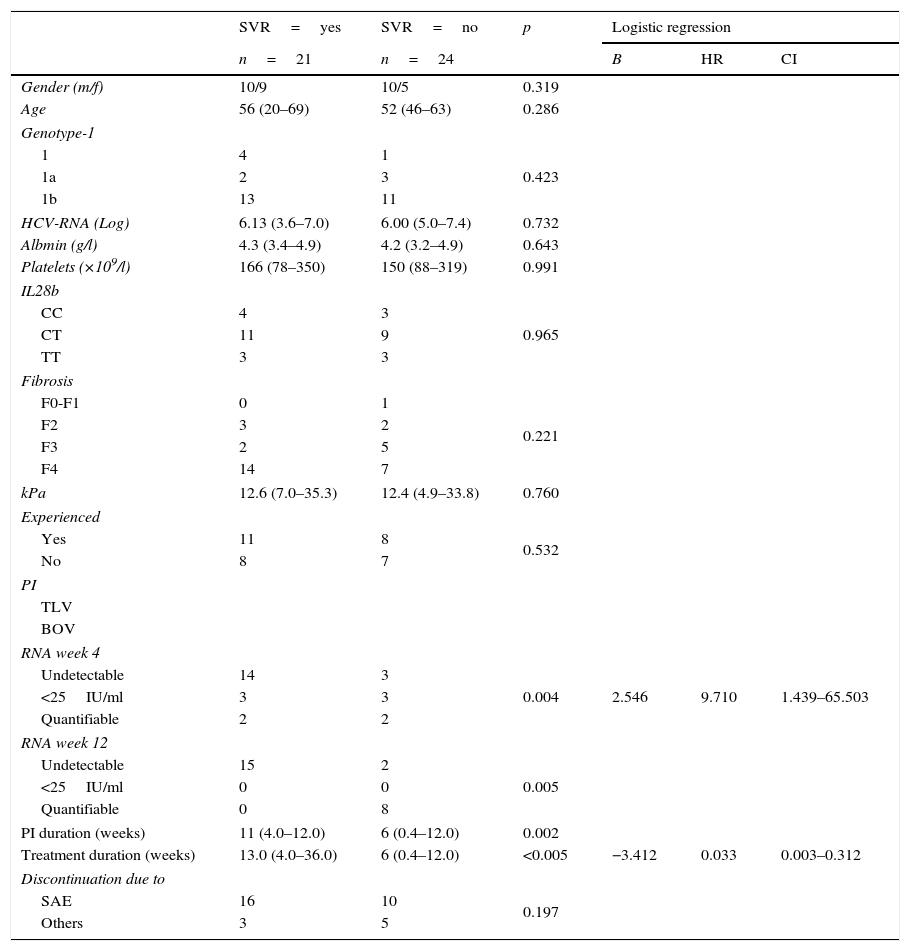
Predictive variables of SVR in index casesTable 2 summarizes the predictive analysis of TLV-treated index cases in terms of the attainment of SVR. The low number of BOV-treated index cases with and without SVR made it impossible to conduct a separate analysis of patients treated with this PI.
Comparative analysis of TLV index cases.
| SVR=yes | SVR=no | p | Logistic regression | |||
|---|---|---|---|---|---|---|
| n=21 | n=24 | B | HR | CI | ||
| Gender (m/f) | 10/9 | 10/5 | 0.319 | |||
| Age | 56 (20–69) | 52 (46–63) | 0.286 | |||
| Genotype-1 | ||||||
| 1 | 4 | 1 | 0.423 | |||
| 1a | 2 | 3 | ||||
| 1b | 13 | 11 | ||||
| HCV-RNA (Log) | 6.13 (3.6–7.0) | 6.00 (5.0–7.4) | 0.732 | |||
| Albmin (g/l) | 4.3 (3.4–4.9) | 4.2 (3.2–4.9) | 0.643 | |||
| Platelets (×109/l) | 166 (78–350) | 150 (88–319) | 0.991 | |||
| IL28b | ||||||
| CC | 4 | 3 | 0.965 | |||
| CT | 11 | 9 | ||||
| TT | 3 | 3 | ||||
| Fibrosis | ||||||
| F0-F1 | 0 | 1 | 0.221 | |||
| F2 | 3 | 2 | ||||
| F3 | 2 | 5 | ||||
| F4 | 14 | 7 | ||||
| kPa | 12.6 (7.0–35.3) | 12.4 (4.9–33.8) | 0.760 | |||
| Experienced | ||||||
| Yes | 11 | 8 | 0.532 | |||
| No | 8 | 7 | ||||
| PI | ||||||
| TLV | ||||||
| BOV | ||||||
| RNA week 4 | ||||||
| Undetectable | 14 | 3 | 0.004 | 2.546 | 9.710 | 1.439–65.503 |
| <25IU/ml | 3 | 3 | ||||
| Quantifiable | 2 | 2 | ||||
| RNA week 12 | ||||||
| Undetectable | 15 | 2 | 0.005 | |||
| <25IU/ml | 0 | 0 | ||||
| Quantifiable | 0 | 8 | ||||
| PI duration (weeks) | 11 (4.0–12.0) | 6 (0.4–12.0) | 0.002 | |||
| Treatment duration (weeks) | 13.0 (4.0–36.0) | 6 (0.4–12.0) | <0.005 | −3.412 | 0.033 | 0.003–0.312 |
| Discontinuation due to | ||||||
| SAE | 16 | 10 | 0.197 | |||
| Others | 3 | 5 | ||||
TLV, telaprevir; BOV, boceprevir; SVR, sustained virological response; B, coefficient; HR, hazard ratio; CI, confidence interval; PI, protease inhibitor.
Patients with and without SVR showed no significant differences regarding demographic variables, genotype-1 subtypes, baseline HCV-RNA, serum albumin, platelets count, IL28B haplotypes, fibrosis scores, previous treatment experience and reasons for interrupting treatment. RNA at week 4 and 12, duration of PI administration and duration of the treatment showed significant differences in the univariate analysis.
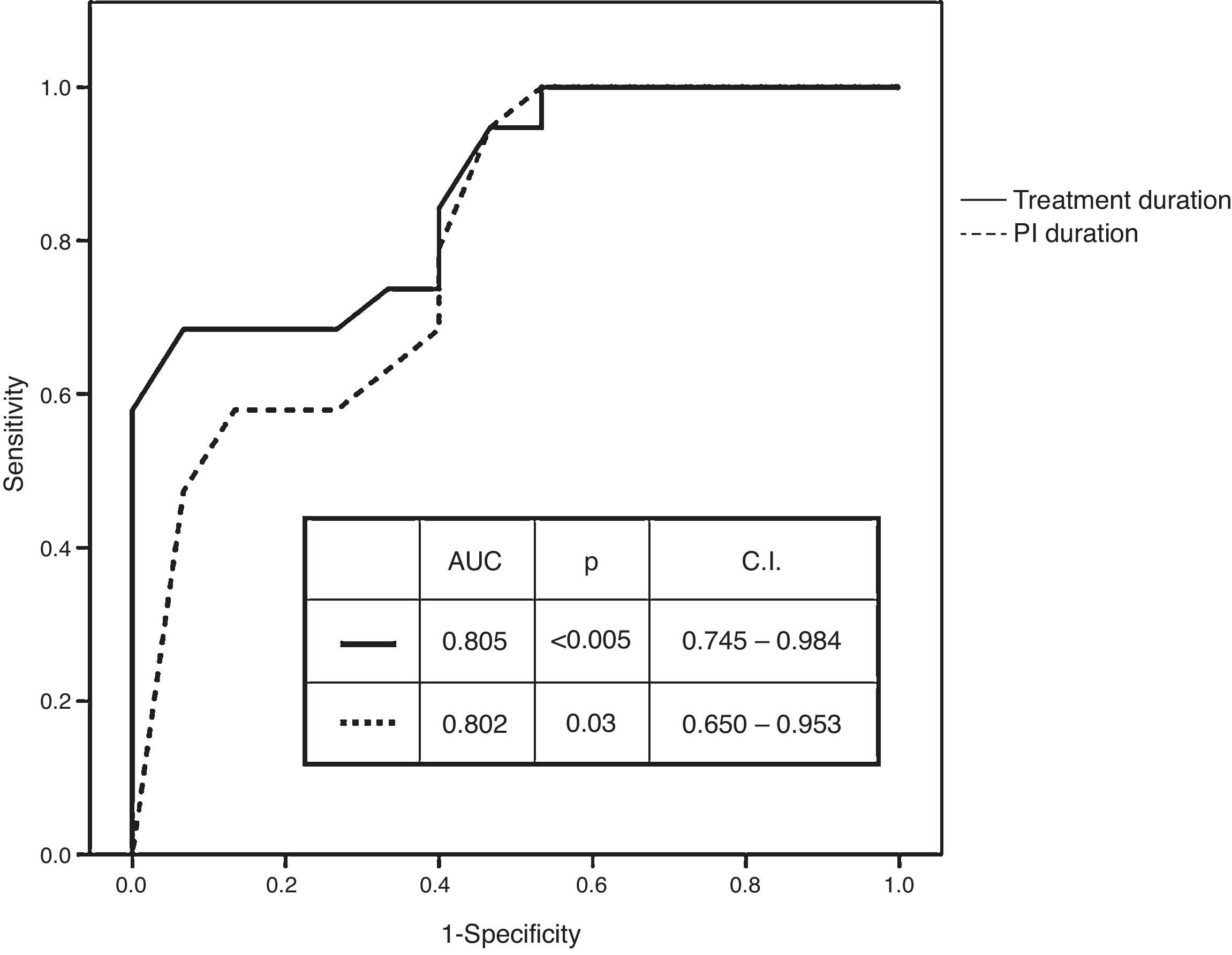
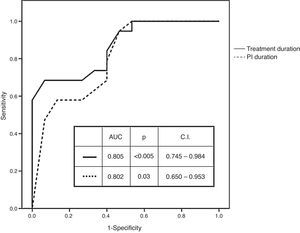
Receiver operating characteristic (ROC) curves of numerical variables that showed significant differences had an AUC of 0.802 (p=0.003; CI: 0.650–0.953) for the duration of PI administration and of 0.865 (p<0.005; CI: 0.746–0.984) for the duration of treatment administration (Fig. 1). Best cut-off (sensitivity versus 1−specificity) levels obtained were 8.5 weeks for the duration of PI administration and of 11 weeks for the duration of the entire treatment protocol respectively.
RNA at treatment week 4 (undetectable, <25IU/ml, quantifiable), RNA at treatment week 12 (undetectable, <25IU/ml, quantifiable), duration of PI administration (different cut-off values ranging from 8 to 11 weeks) and treatment duration (different cut-off values ranging from 8 to 13 weeks) were introduced in stepwise logistic regression models. RNA at treatment week 4 (HR: 9.710; CI: 1.439–65.503) and treatment duration (≤11 versus >11 weeks) (HR: 0.033; CI: 0.003–0.312) were the variables with independent predictive value.
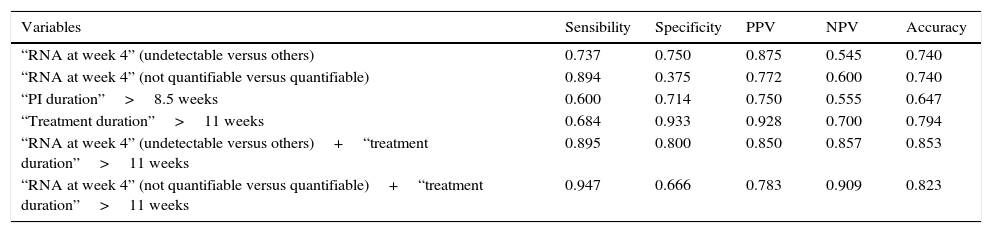
The analysis of the prognostic value of variables that showed significant differences for SVR in index cases focused only on the 34 index cases treated with TLV (Table 3). As individual variables, treatment duration longer than 11 weeks showed the best accuracy (0.794) with very high values of specificity (0.933) and PPV (0.928) but with a sensitivity of only 0.684. The association of HCV-RNA at treatment week 4 (undetectable versus detectable or quantifiable) and/or a duration of the treatment longer than 11 weeks in every index case considerably improved all the prognostic parameters with a very high degree of homogeneity.
Predictive analysis in TLV-treated index cases.
| Variables | Sensibility | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| “RNA at week 4” (undetectable versus others) | 0.737 | 0.750 | 0.875 | 0.545 | 0.740 |
| “RNA at week 4” (not quantifiable versus quantifiable) | 0.894 | 0.375 | 0.772 | 0.600 | 0.740 |
| “PI duration”>8.5 weeks | 0.600 | 0.714 | 0.750 | 0.555 | 0.647 |
| “Treatment duration”>11 weeks | 0.684 | 0.933 | 0.928 | 0.700 | 0.794 |
| “RNA at week 4” (undetectable versus others)+“treatment duration”>11 weeks | 0.895 | 0.800 | 0.850 | 0.857 | 0.853 |
| “RNA at week 4” (not quantifiable versus quantifiable)+“treatment duration”>11 weeks | 0.947 | 0.666 | 0.783 | 0.909 | 0.823 |
PPV, positive predictive value; NPV, negative predictive value; PI, protease inhibitor.
This retrospective study confirms that after early discontinuation of triple therapy with TLV for reasons other than stopping rules, the rate of SVR remains significant (55.9% in our study). The SVR rate recorded was similar to the highest ones obtained in the pivotal PROVE-1, -2, -3 and ILLUMINATE studies of TLV in patients who had discontinued the treatment for any reason.3–5,8
Our therapeutic cohort study including 467 patients was carried out within the restrictions for the use of first generation protease inhibitors in Spain.1,2 As these restrictions considered patients with advanced disease (F3 and F4) a priority as to treatment, a lower tolerability and a high rate of treatment discontinuation were to be expected. However, the treatment discontinuation rate recorded (21.1%) was no higher than those found in the TLV12PR48 arms of TLV pivotal phase III studies, which ranged from 19.2% to 42.3%.6–8 As a matter of fact, the discontinuation rates found in pivotal studies of TLV make it unlikely that the occurrence of a significant SVR in early discontinuers for reasons other than stopping rules had gone unrecognized. As SVR was mentioned in early discontinuers in some of the pivotal studies,3–5,8 it is striking that no effort has been made so far to reflect on the characteristics of these subset of patients. On the other hand, our index cases treated with BOV presented very low levels of SVR, and so, the lack of comment in the literature regarding these patients is less surprising.
TLV and BOV treatments showed differences in duration of time-to-interruption (shorter with TLV) and in reasons for discontinuation (more likely due to stopping rules with BOV and more likely due to reasons other than stopping rules with TLV). TLV and BOV are drugs with entirely different schedules of administration and with futility rules spreading over different periods of time. This makes it impossible to compare them in terms of the same frame of time-limited prognostic variables. BOV-treated patients are thus an entirely different cohort requiring a separate prognostic study. The fact that only 11 BOV-treated index cases were included in this cohort, and that only 2 of them attained SVR, prevent us from expanding on this subset of patients or from drawing any further conclusion.
Although the median treatment duration in index cases that attained SVR was around 13 weeks, a few patients required only 4 or 6 weeks of TLV-based triple therapy. These cases highlight the value of having prognostic tools to predict treatment success or failure when a patient discontinues the treatment while presenting a good response. In this study, the variables that may explain the attainment of SVR with shorter-than-scheduled TLV treatments were a rapid fall in viral load at week 4 (undetectable or <25IU/ml) and a treatment of sufficient duration. The fact that logistic regression analysis selected both variables indicates that both are necessary as predictive factors (Table 3). These are the same requirements included in the definition of eRVR, i.e., undetectable HCV-RNA at treatment weeks 4 and 12. eRVR was shown to be such a powerful predictive factor of SVR in the pivotal phase III of TLV6–8,13 that it performed better than other predictors of response used previously such as pre-treatment HCV-RNA.6,7
This study offers several predictive values in the specific setting of what we defined as index cases. Medians of 11 weeks for the duration of PI administration and 13.5 weeks for duration of the treatment as well as cut-off (sensitivity/1−specificity) values of 8.5 and >11 weeks for the same predictive variables are the most relevant. Total treatment duration (<11 weeks versus >11 weeks) performed better than the duration of PI administration for every predictive parameter (Table 3), indicating the importance of fulfilling the 12-week triple phase of the PRTLV protocol. A higher predictive power can be obtained by combining the 2 variables selected in the logistic regression analysis, HCV-RNA (undetectable versus detectable or quantifiable) and or a treatment duration (<11 weeks versus >11 weeks). Fulfilment of one or both conditions in each single index case made it possible to substantially improve the predictive power of the variables, with a sensitivity of 0.895 and a specificity of 0.800.
The presence of TLV-treated patients who attained SVR after only 4 and 6 weeks of treatment indicates that there may be other variables not identified in the study that can predict SVR. It may also be that because of the small number of TLV-treated index cases, it was impossible to detect any predictive role for other variables included in the study such as fibrosis scores, IL28B haplotypes, previous treatment experience or the ability of some SAE to modulate the immune response and treatment outcome. The analysis of other markers and/or polymorphisms proposed as predictive factors in previous studies,14–16 lies beyond the scope of this clinical study in the real world setting.
The retrospective nature of the study and the inter-centre variability of the compiled data mean that our conclusions need to be validated by other independent cohorts. In any case, the emergence of direct antiviral drugs and interferon-free treatments make it unlikely that further prognostic studies with first generation PI will be carried out.
In summary, this study confirms that after early discontinuation of triple therapy with TLV due to reasons other than stopping rules, the SVR rate remains significant. This favourable outcome did not apply to BOV-treated patients which represent an entirely different cohort requiring an independent prognostic study. Undetectable HCV-RNA at week 4 of treatment and a treatment duration longer than 11 weeks are strongly predictive of SVR in this subset of TLV-treated patients. These results may help improve the prognostic approach and management of patients after early discontinuation of triple therapy with first generation PI in countries in which they are still prescribed.
Financial supportNone.
Conflict of interestsAuthors reported no conflict of interests in relation to this study.
We thank Silvia Virolés Torrent, Lourdes Martos García, Gloria de la Red Bellvis, Esther Niño Aragón and Cinta Cardona Castellà for their help in compiling the survey data and the patient charts at their hospitals.