Real-world evidence (RWE) on vedolizumab (VDZ), an anti-lymphocyte trafficking treatment that selectively targets the α4β7/MAdCAM-1 interaction on the gut, is mostly limited to patients who are repeatedly refractory to anti-tumor necrosis factor-alpha (anti-TNF-α), and other treatments. The EVOLVE-IBERIA study assessed VDZ or anti-TNF-α as first- or second-line biologic treatment, in patients with Crohn's disease or ulcerative colitis (UC); here, we present the outcomes in patients with UC.
Patients and methodsMedical records were retrospectively reviewed from 25 hospitals in Spain and Portugal. Eligible patients with UC were aged ≥18 years and had received treatment with first- or second-line VDZ or anti-TNF-α. Objectives were to evaluate clinical effectiveness, safety, and treatment patterns of VDZ and anti-TNF-α, and to characterize healthcare resource utilization. Baseline covariates were balanced in both cohorts by means of propensity scores, using the inverse probability of treatment weighting (PS-IPTW) method.
ResultsA total of 199 patients with UC were included (median follow-up: 24.0 months). At Week 52, clinical response rates were 75.6% and 73.2% (p=0.72) and clinical remission rates were 56.6% and 62.0% (p=0.49), in the VDZ cohort and anti-TNF-α cohort, respectively. Treatment-related adverse event rates per 100 patient-years were 0.23 in the VDZ cohort and 1.1 in the anti-TNF-α cohort (p=0.037).
ConclusionThe similar long-term effectiveness and lower incidence of adverse events of VDZ compared with anti-TNF-α in the real-world setting, confirm the favorable benefit:risk ratio of VDZ as first- or second-line biologic treatment for UC.
La evidencia en práctica clínica real sobre vedolizumab (VDZ), un tratamiento antitráfico linfocitario selectivo a nivel intestinal, se limita a pacientes refractarios a tratamiento previo con inhibidores del factor de necrosis tumoral alfa (anti-TNF-α). Este estudio evaluó VDZ en comparación con anti-TNF-α como tratamiento biológico de primera o segunda línea en pacientes con enfermedad de Crohn y colitis ulcerosa (CU). Presentamos los resultados en pacientes con CU.
Pacientes y métodosSe revisaron retrospectivamente las historias clínicas de pacientes de 25 hospitales en España y Portugal. Los pacientes elegibles tenían≥18 años y CU, habiendo recibido VDZ o anti-TNF-α como terapia biológica de primera o segunda línea. Se analizaron la efectividad clínica, la seguridad, los patrones de tratamiento y la utilización de recursos sanitarios. Para equilibrar las covariables basales, se aplicaron puntuaciones de propensión mediante el método de ponderación por probabilidad inversa de tratamiento.
ResultadosSe incluyeron 199 pacientes con CU (mediana de seguimiento: 24,0 meses). En la semana 52, las tasas de respuesta clínica fueron 75,6 y 73,2% (p=0,72), y las tasas de remisión clínica fueron 56,6 y 62,0% (p=0,49), en la cohorte de VDZ y de anti-TNF-α, respectivamente. Las tasas de eventos adversos relacionados con el tratamiento por cada 100 pacientes-año fueron 0,23 en la cohorte de VDZ y 1,1 en la de anti-TNF-α (p=0,037).
ConclusiónLa efectividad similar a largo plazo y la menor incidencia de eventos adversos de VDZ en comparación con los anti-TNF-α respaldan el perfil beneficio-riesgo favorable de VDZ como tratamiento biológico de primera o segunda línea para el tratamiento de la CU.
Inflammatory bowel disease (IBD), inclusive of Crohn's disease and ulcerative colitis (UC), is a chronic, debilitating condition that affects the gastrointestinal tract and is characterized by a progressive disease course.1,2 The prevalence of IBD is increasing worldwide; IBD has been shown to affect approximately 0.2% of the European population.3 Within Europe, the prevalence of IBD varied substantially within regions, ranging from 136.6 per 100,000 persons in Western Europe to 104.5 per 100,000 persons in Eastern Europe.3 Drug-related expenditures and substantial indirect costs arising from the loss of productivity have resulted in a high economic burden associated with IBD across Europe.3 In Spain, estimated prevalence of IBD is 88.7 per 100,000 persons in 2011.4,5 However, other studies with a regional scope reported a higher prevalence (>200 per 100,000 persons) in the same year, and an increasing trend over time.4,6,7 In Portugal, the estimated prevalence of IBD is 146 per 100,000 persons in 2007.8
The prevalence of UC in Europe varies from 2.4 to 294 cases per 100,000 persons, with approximately 2.1 million persons with UC in Europe.9 In Spain, the prevalence of UC, is estimated at 89 cases per 100,000 persons.5 In Portugal, the prevalence of UC, is estimated at 71 cases per 100,000 persons.8
Patients with UC experience periods of relapse and remission, and early relapse or active disease in the first 2years is associated with worse disease outcomes subsequently.10 A systematic review based on medical records of 750 patients with UC diagnosed in Oxford (UK), suggests that approximately 25% of all patients with UC will experience at least one episode of acute severe colitis and 12% of patients with UC will require a colectomy.11
According to the European Crohn's and Colitis Organisation (ECCO) guidelines, patients with mild-to-moderately active UC should be treated initially with 5-aminosalicylic (5-ASA) therapy.12 Patients with mild-to-moderate UC in whom 5-ASA induction therapy fails or is not tolerated should be treated with oral prednisolone.12 Alternatively, topically acting oral corticosteroids such as budesonide multimatrix and beclomethasone dipropionate can be used as treatments for those wishing to avoid systemic corticosteroids.12 The ECCO guidelines suggest that patients with moderate-to-severe UC should be treated with oral corticosteroids such as prednisolone; however, given the potential for side effects, some of which are irreversible, corticosteroid-free remission represents a desired outcome for patients with UC.12 Additionally, courses of corticosteroids should be restricted to a maximum of 3 months. Patients that do not respond to conventional therapy should be considered for treatment escalation to biologics.12 Vedolizumab (VDZ), can be used in the induction and maintenance of remission of UC in patients where conventional treatment has failed.12 VDZ is an anti-α4β7 integrin monoclonal antibody, which reduces gut inflammation by inhibiting the binding of α4β7 to the mucosal addressin cell adhesion molecule-1 (MAdCAM-1).13
The GEMINI 1 trial assessed the efficacy of VDZ versus placebo as induction and maintenance therapy for UC.14 Data from the GEMINI 1 trial demonstrated that glucocorticoid-free remission at Week 52 was achieved in 31.4% of the patients who received VDZ every 8 Weeks and in 45.2% of those who received VDZ every 4 Weeks, as compared with 13.9% of patients who received placebo.14 In May 2014, VDZ received approval by the European Medicines Agency for the treatment of moderate-to-severe UC,15,16 and in May 2020 a subcutaneous administration of VDZ received approval for maintenance therapy in adults with moderately-to-severely active UC in European Union.17
Although a head-to-head trial demonstrating superiority of VDZ versus adalimumab for moderate-to-severe UC exist,18 real-world data on VDZ to date have focused primarily on highly anti-tumor necrosis-α (anti-TNF-α) refractory cohorts. This study addresses a need for real-world evidence (RWE) and comparative data obtained in earlier treatment lines.
The objectives of the EVOLVE-IBERIA study (NCT03710486) were to compare the clinical effectiveness in the real-world setting, safety, treatment patterns and healthcare resource utilization of VDZ and anti-TNF-α. This study aimed to generate additional RWE for VDZ and anti-TNF-α as first- or second-line biologic treatment, in patients with Crohn's disease or UC; here we present the outcomes in patients with UC.
Materials and methodsObjectivesThe clinical effectiveness, safety and treatment patterns of patients treated with VDZ and anti-TNF-α when used as first- or second-line biologic for the treatment of UC are described. The healthcare resource utilization (including healthcare professional and emergency department visits, hospitalizations, and IBD-related surgical procedures) are quantified.
Study endpoints are listed in the Supplemental Information.
Study designBetween January 2019 and February 2022 medical charts from patients with UC in 25 centers (20 in Spain and 5 in Portugal) were retrospectively evaluated. Demographics and medical history disease activity related to UC (clinical, endoscopic, and biochemical outcomes), dose increases (intensifications), comorbid medical conditions, concomitant use of non-biologic drugs, treatment withdrawals, switching of biologic treatment, and adverse events (AEs) data were collected.
To avoid bias in the selection of patients, centers were instructed to start data abstraction after selecting a random sample of eligible patients. Each center followed a 4-block procedure in which patients were stratified according to the type of treatment (i.e., VDZ vs. anti-TNF-α) and line of treatment (first or second biologic), to have a similar number of patients per block. Furthermore, efforts were made to increase the relevance of the data to the general population, by including centers that varied by geographical location and institution type.
PatientsPatients aged 18 years or older at the time of treatment initiation with moderate-to-severe UC who initiated first- or second-line treatment with VDZ or an anti-TNF-α agent during the eligibility period were included.
Full study eligibility criteria are described in the Supplemental Information.
Clinical effectivenessClinical response and remission were assessed using the partial Mayo score and physician assessment. Clinical response was defined as either a decrease in partial Mayo score ≥2 or by physician assessment of clinical response. Clinical remission was defined as a partial Mayo score ≤2 or by physician assessment of clinical remission.
Endoscopic response and remission were assessed by physician assessment.
Clinical assessment of biochemical remission was based on the measured levels (mg/L) of C-reactive protein (CRP) or on the measured levels of fecal calprotectin. Biochemical remission was defined as a level of CRP<5mg/L or as a level of fecal calprotectin of 250μg/g.
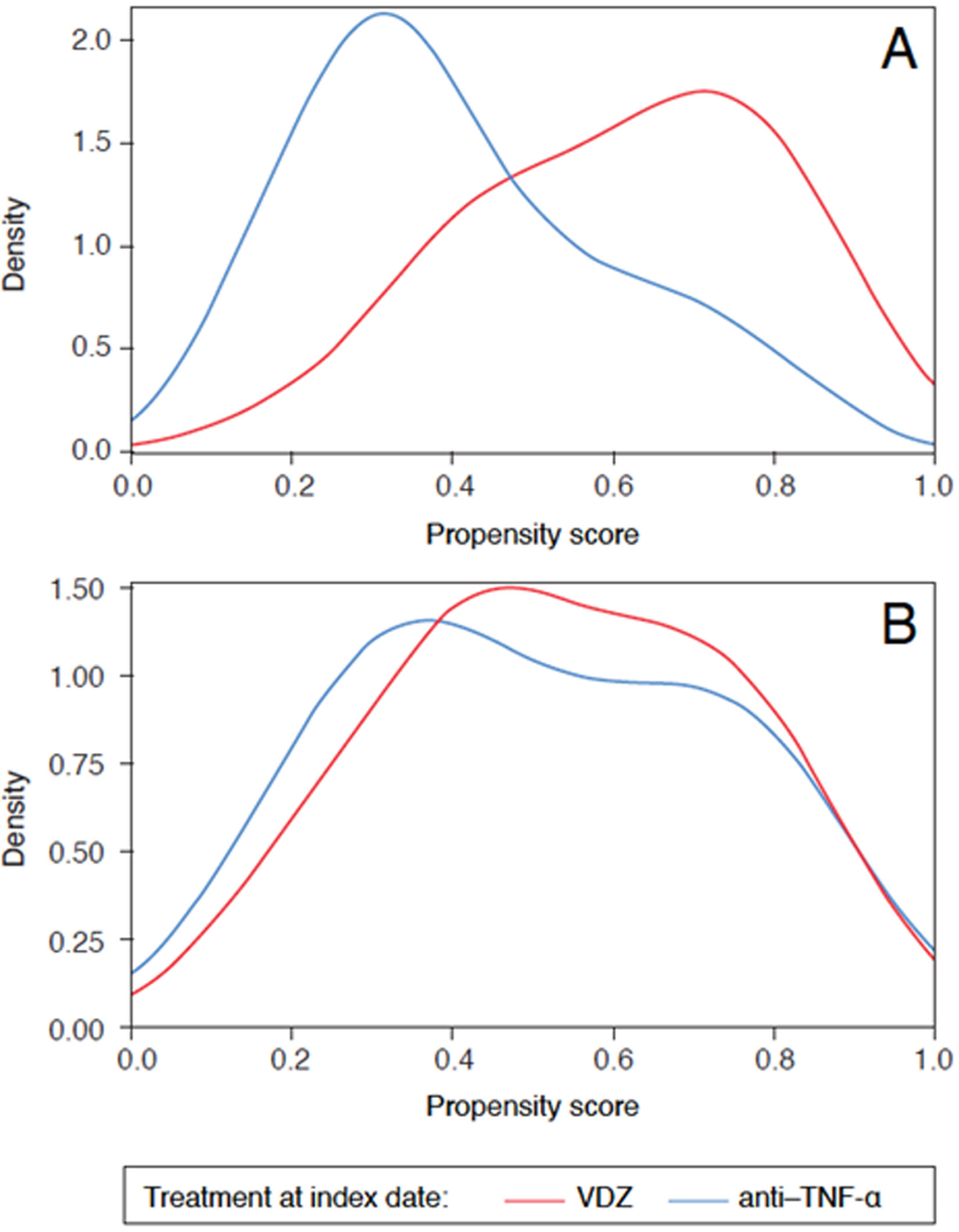
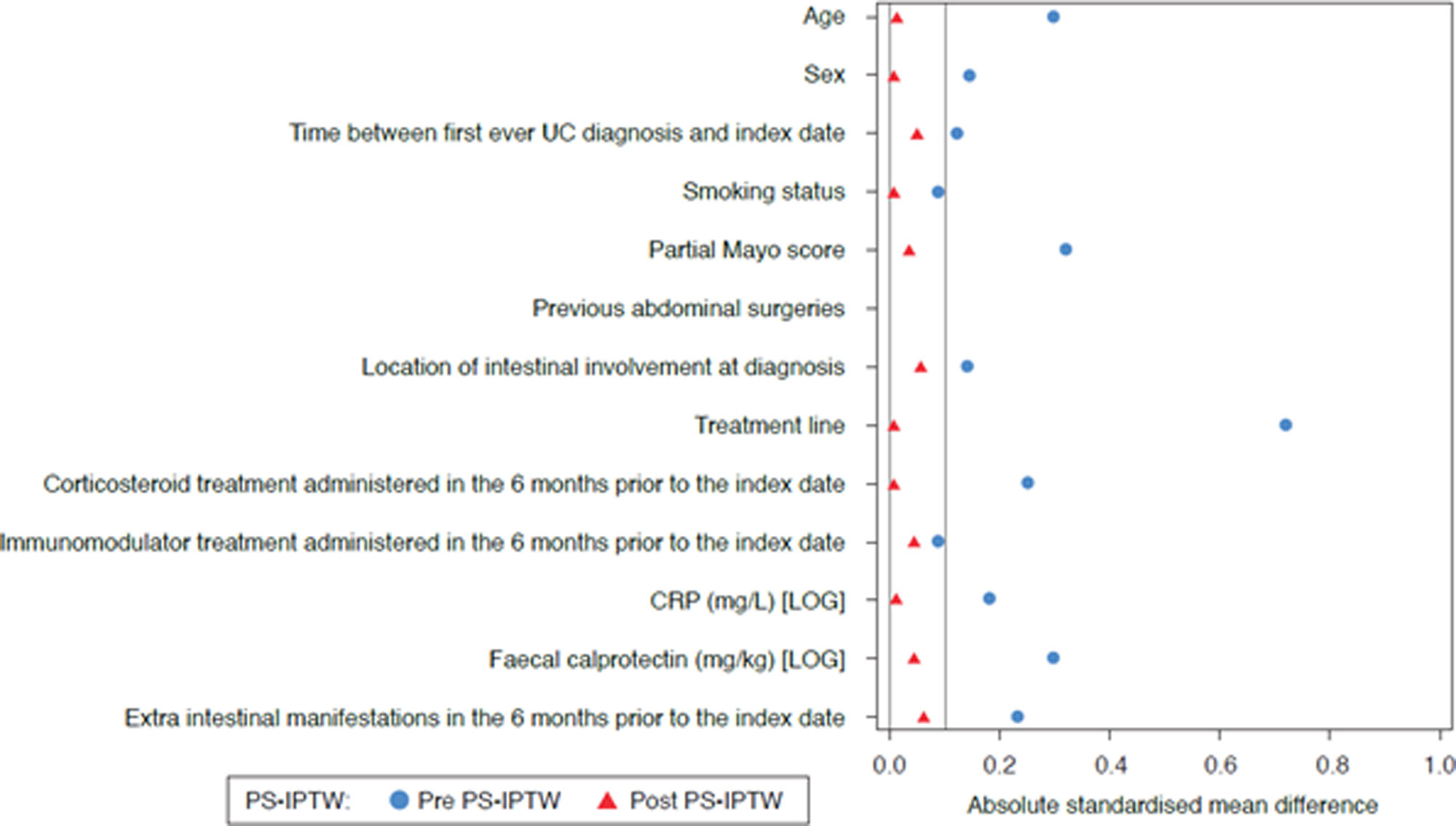
StatisticsA list of endpoints is provided in the Supplemental Information section. To avoid bias in the interpretation of treatment effectiveness and safety, both cohorts were balanced regarding baseline clinical and demographic parameters by means of propensity scores, using the inverse probability of treatment weighting (PS-IPTW) method. The covariates balance between both cohorts achieved after PS-IPTW was evaluated by means of density plots and LOVE (LOcal Variance of Effect) plots. A logistic regression with treatment group as a response has been adjusted to estimate PS-IPTW. The analysis parameters included in the model are shown in the Supplemental Information section.
Statistical analysis was performed using SAS® software (version 9.4).20
Statistical significance of the differences between cohorts was evaluated by means of t- and Chi-square tests. The bilateral level of significance for all tests was 0.05. Time-to-event analyses (e.g., time to first dose escalation, time to discontinuation) are represented by means of Kaplan–Meier curves.
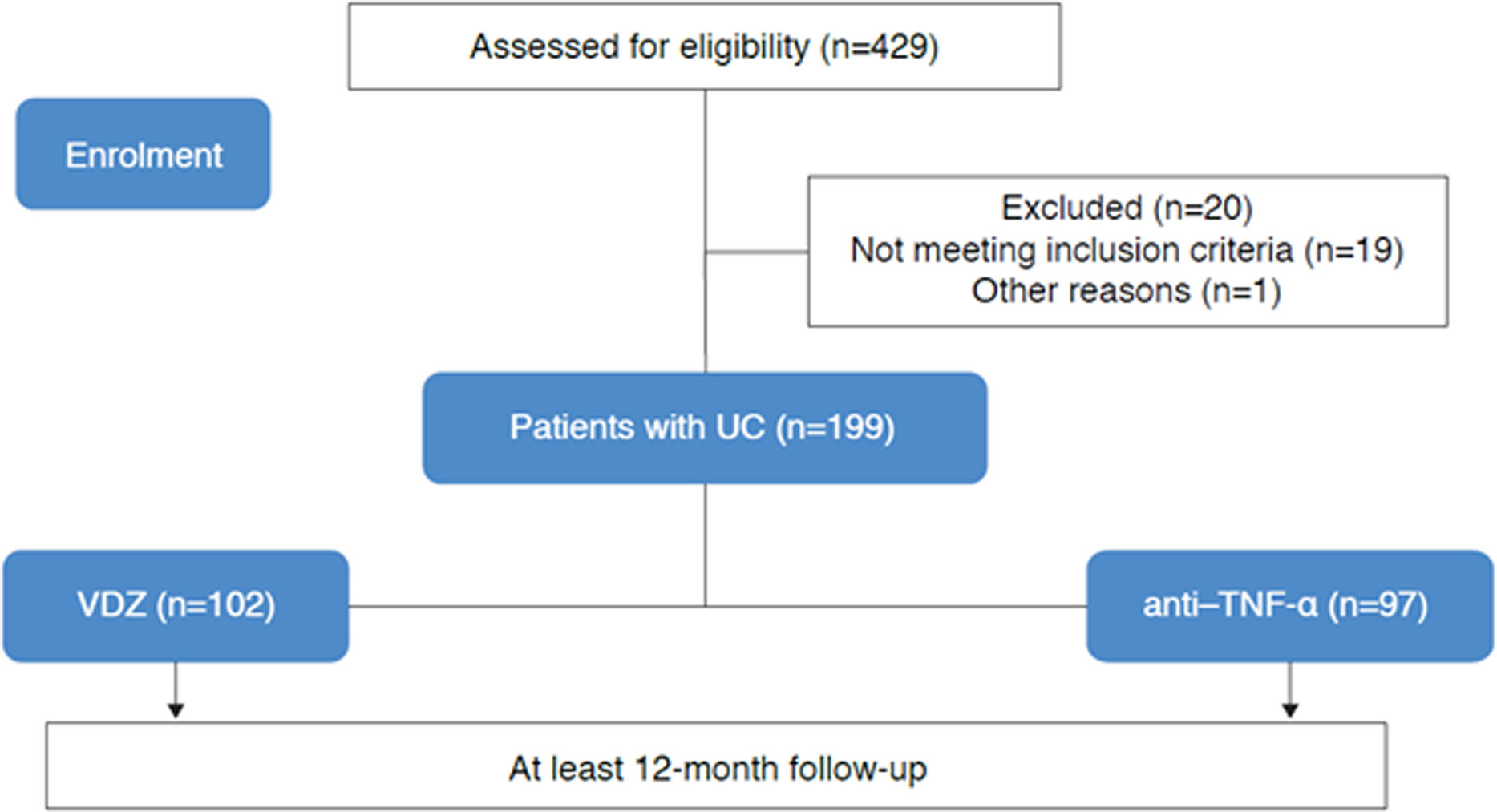
ResultsBaseline characteristics and demographicsIn total, 199 patients with UC were included in the study; baseline characteristics and demographic information pre- and post-PS-IPTW are summarized in Table 1. Baseline covariates resulted appropriately balanced in both cohorts after PS-IPTW, as shown in Figs. 1 and 2. Overall, 102 patients started VDZ treatment (44.1% were bio-naïve), and 97 patients started anti-TNF-α treatment (77.3% were bio-naïve) (Fig. 3). Within this cohort, 43.3% received biosimilar infliximab, 24.7% received original adalimumab, 20.6% received original golimumab, 8.2% received original infliximab, and 3.1% received biosimilar adalimumab. The median follow-up was 23.7 months (95% confidence interval: 17.9–30.8).
Baseline characteristics.
| Pre PS-IPTW | Post PS-IPTW | |||
|---|---|---|---|---|
| VDZ | Anti-TNF-α | VDZ | Anti-TNF-α | |
| Age at index date (years), mean (SE) | 53.1 (1.93) | 47.8 (1.74) | 51.5 (1.89) | 51.3 (1.70) |
| Sex, n (%) | ||||
| Male | 69 (67.6) | 59 (60.8) | 68 (67.1) | 65 (67.4) |
| Time between first ever UC diagnosis and index date (years), mean (SE) | 8.4 (0.9) | 9.4 (1.0) | 9.6 (1.0) | 9.2 (1.0) |
| Smoking status at index treatment initiation, n (%) | ||||
| Never smoked | 52 (51.1) | 53 (54.5) | 49 (47.9) | 46 (47.0) |
| Former smoker | 42 (41.6) | 39 (40.1) | 47 (46.1) | 46 (47.7) |
| Current smoker | 7 (7.3) | 5 (5.4) | 6 (6.0) | 5 (5.3) |
| Partial Mayo score, mean (SE) | 4.0 (0.3) | 4.8 (0.3) | 4.2 (0.3) | 4.3 (0.3) |
| Previous abdominal surgeries, n (%) | ||||
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Location of intestinal involvement at diagnosis, n (%) | ||||
| Proctitis/left colitis | 60 (59.2) | 51 (52.3) | 58 (56.8) | 58 (59.6) |
| Extensive colitis | 42 (40.8) | 46 (47.7) | 44 (43.2) | 39 (40.4) |
| Treatment line, n (%) | ||||
| First biologic (bio-naïve) | 45 (44.1) | 75 (77.3) | 61 (59.4) | 57 (59.0) |
| Second biologic | 57 (55.9) | 22 (22.7) | 41 (40.6) | 40 (41.0) |
| Any corticosteroids treatment administered within the 6 months prior to the index date, n (%) | ||||
| Yes | 57 (55.9) | 66 (68.0) | 58 (57.2) | 56 (57.5) |
| Any immunomodulators treatment administered within the 6 months prior to the index date, n (%) | ||||
| Yes | 42 (41.2) | 44 (45.4) | 44 (43.4) | 40 (41.3) |
| CRP (mg/L) [LOG], mean (SE) | 0.9 (0.2) | 1.3 (0.2) | 1.1 (0.2) | 1.1 (0.2) |
| Fecal calprotectin (mg/kg) [LOG], mean (SE) | 6.3 (0.2) | 6.7 (0.2) | 6.4 (0.2) | 6.5 (0.22) |
| The patient had extra-intestinal manifestations within 6 months prior to the index date, n (%) | ||||
| Yes | 9 (8.8) | 16 (16.5) | 16 (16.5) | 13 (13.1) |
CRP, C-reactive protein; PS-IPTW, propensity scores inverse probability of treatment weighting; SE, standard error; TNF-α, tumor necrosis factor-alpha; UC, ulcerative colitis; VDZ, vedolizumab.
Density plots before (A) and after (B) PS-IPTW. Distribution of the propensity scores among patients with UC for the VDZ cohort (red) and for the anti-TNF-α cohort (blue), before (A) and after (B) PS-IPTW. PS-IPTW, propensity scores inverse probability of treatment weighting; TNF-α, tumor necrosis factor-alpha; VDZ, vedolizumab.
LOVE plot of standardized differences before and after PS-IPTW. Standardized differences for each baseline characteristic among patients with UC between the VDZ and the anti-TNF-α cohort, before (blue) and after (red) PS-IPTW. CRP, C-reactive protein; PS-IPTW, propensity scores inverse probability of treatment weighting; UC, ulcerative colitis.
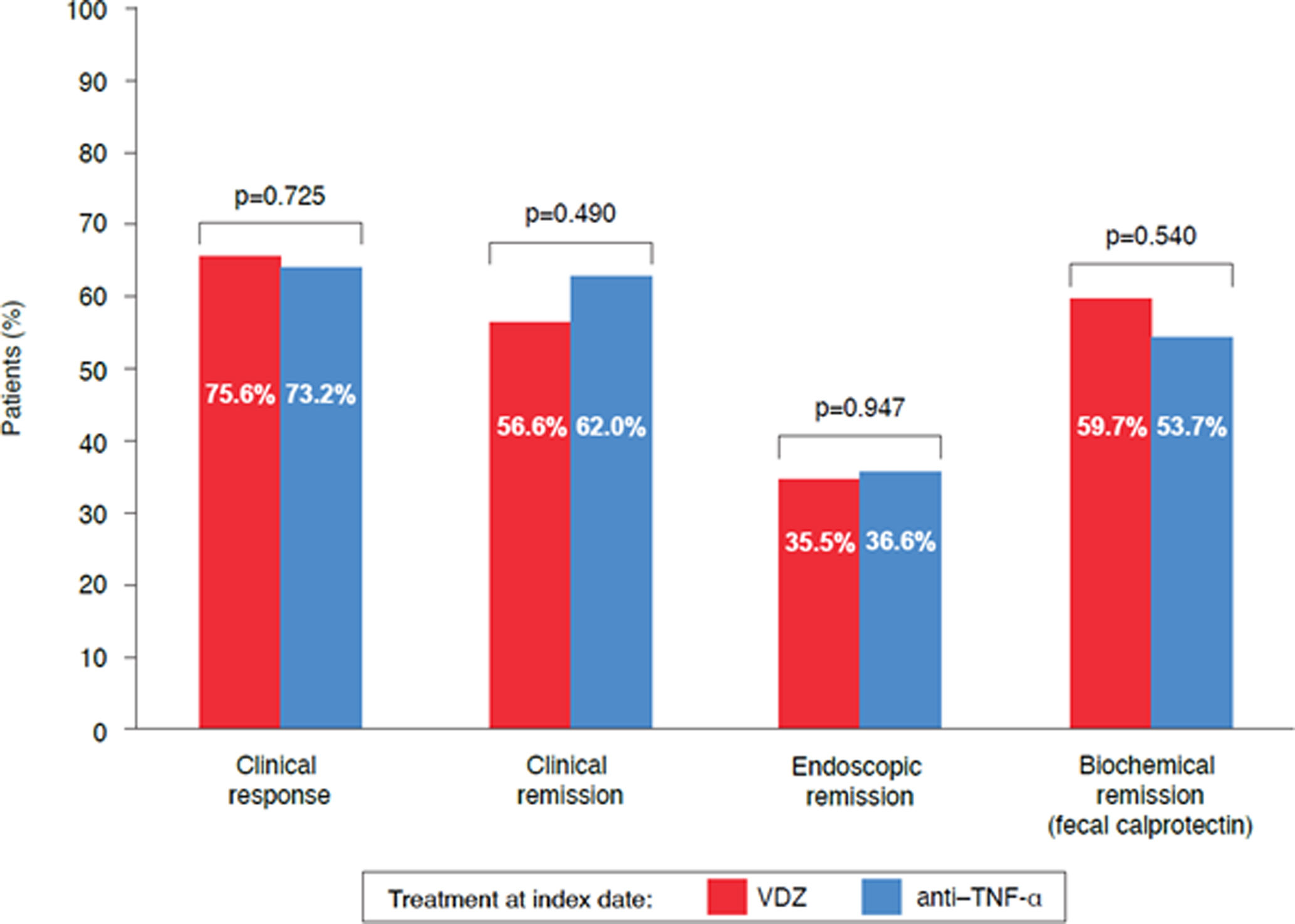
At Week 14, clinical response rates were 81.2% in the VDZ cohort and 80.5% anti-TNF-α cohort (p=0.915). At Week-52, clinical response rates were 75.6% in the VDZ cohort and 73.2% in the anti-TNF-α cohort (p=0.725; Fig. 4).
Effectiveness at Week 52. Rates, expressed as percentages, of clinical response, clinical remission, endoscopic remission and biochemical remission among patients with UC in the VDZ (red) and anti-TNF-α (teal) cohorts. Differences between cohorts were compared by means of t-tests, with a bilateral significance level of 0.05. CRP, C-reactive protein; TNF-α, tumor necrosis factor-alpha; UC, ulcerative colitis, VDZ, vedolizumab.
At Week-14, clinical remission rates were 52.2% in the VDZ cohort and 54.7% in the anti-TNF-α cohort (p=0.763). At Week 52, clinical remission rates were 56.6% in the VDZ cohort and 62.0% in the anti-TNF-α cohort (p=0.490; Fig. 4).
At Week 52, endoscopic response rates were 44.8% in the VDZ cohort and 78.2% in the anti-TNF-α cohort (p=0.028). At Week 52, endoscopic remission rates were 35.5% in the VDZ cohort and 36.6% in the anti-TNF-α cohort (p=0.947; Fig. 4).
At Week 14, biochemical remission rates, based on CRP<5mg/L, were 58.5% and 77.2% in the VDZ cohort and in the anti-TNF-α cohort, respectively (p=0.010). Week 52, biochemical remission rates based on CRP were 60.4% and 85.2% in the VDZ cohort and in the anti-TNF-α cohort, respectively (p=0.001).
At Week 14, biochemical remission rates, based on fecal calprotectin<250μ/g, were 44.7% in the VDZ cohort and 36.8% in the anti-TNF-α cohort (p=0.403). At Week 52, biochemical remission rates (based on fecal calprotectin<250μ/g) were 59.7% in the VDZ cohort and 53.7% in the anti-TNF-α cohort (p=0.540; Fig. 4).
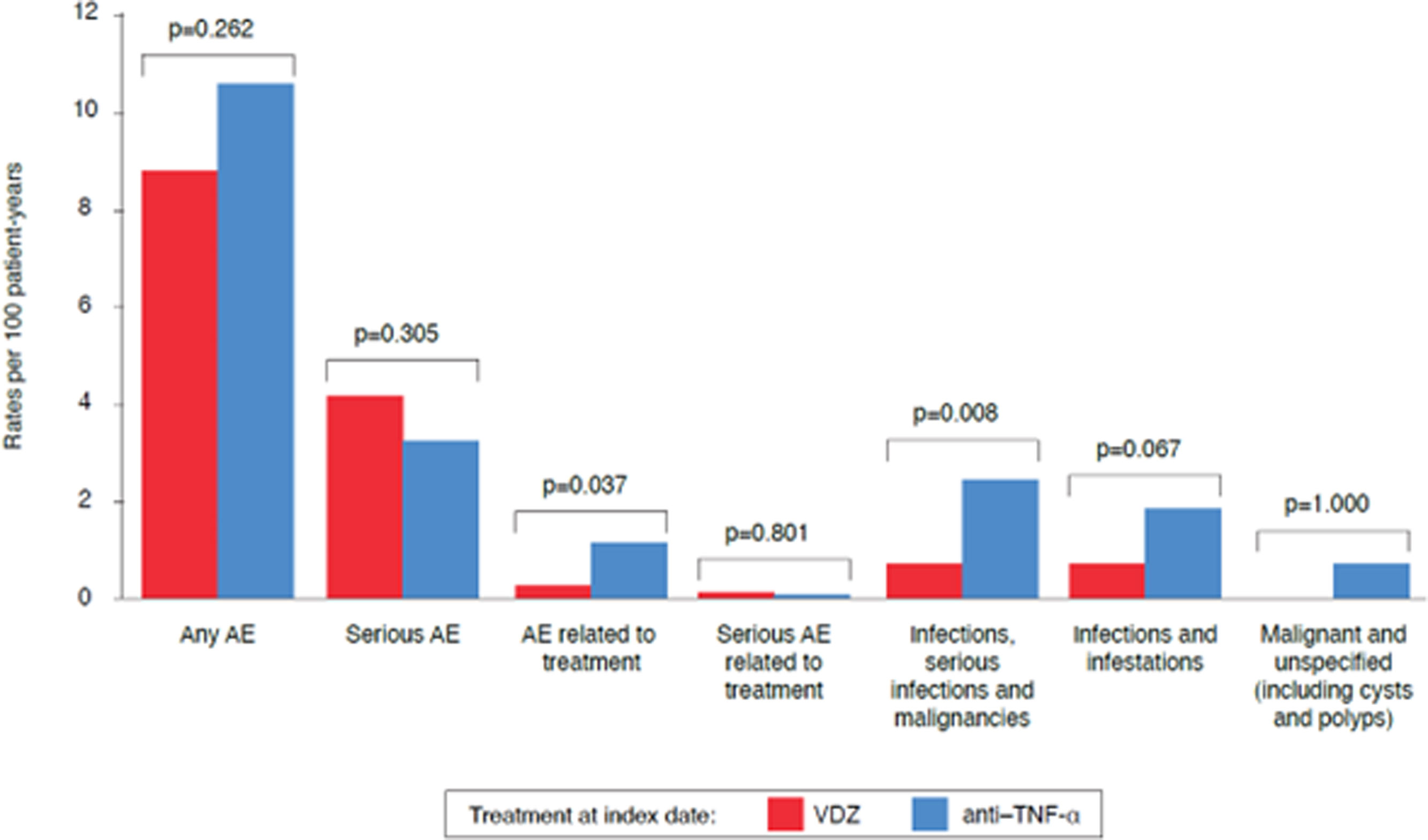
SafetySafety data are summarized in Fig. 5 and Table S1; AE rates per 100 patient-years, pre- and post-PS-IPTW are described. AEs of any type were 8.83 per 100 patient-years in the VDZ cohort and 10.64 per 100 patient-years in the anti-TNF-α cohorts (p=0.262). Serious AEs were reported in 4.17 per 100 patient-years in the VDZ cohort and 3.21 per 100 patient-years in the anti-TNF-α cohort (p=0.305). Treatment-related AEs incidences were 0.23 per 100 patient-years in the VDZ cohort and 1.1 per 100 patient-years in the anti-TNF-α cohort versus (p=0.037). The rates of serious treatment-related AEs were 0.09 per 100 patient-years in the VDZ cohort and 0.06 per 100 patient-years in the anti-TNF-α cohort (p=0.801). The rates of AEs of special interest, including infections, serious infections, and malignancies, were 0.75 per 100 patient-year in the VDZ cohort and 2.4 per 100 patient-year in the anti-TNF-α cohort (p=0.008) (Fig. 5).
Safety outcomes (incidence rates after PS-IPTW adjustment). Incidence rates, expressed as 100 patient-years, of safety outcomes among patients with UC in the VDZ (red) and anti-TNF-α (teal) cohorts. Differences between cohorts were compared by means of t-tests, with a bilateral significance level of 0.05. AE, adverse event; PS-IPTW, propensity scores inverse probability of treatment weighting; TNF-α, tumor necrosis factor-alpha; UC, ulcerative colitis; VDZ, vedolizumab.
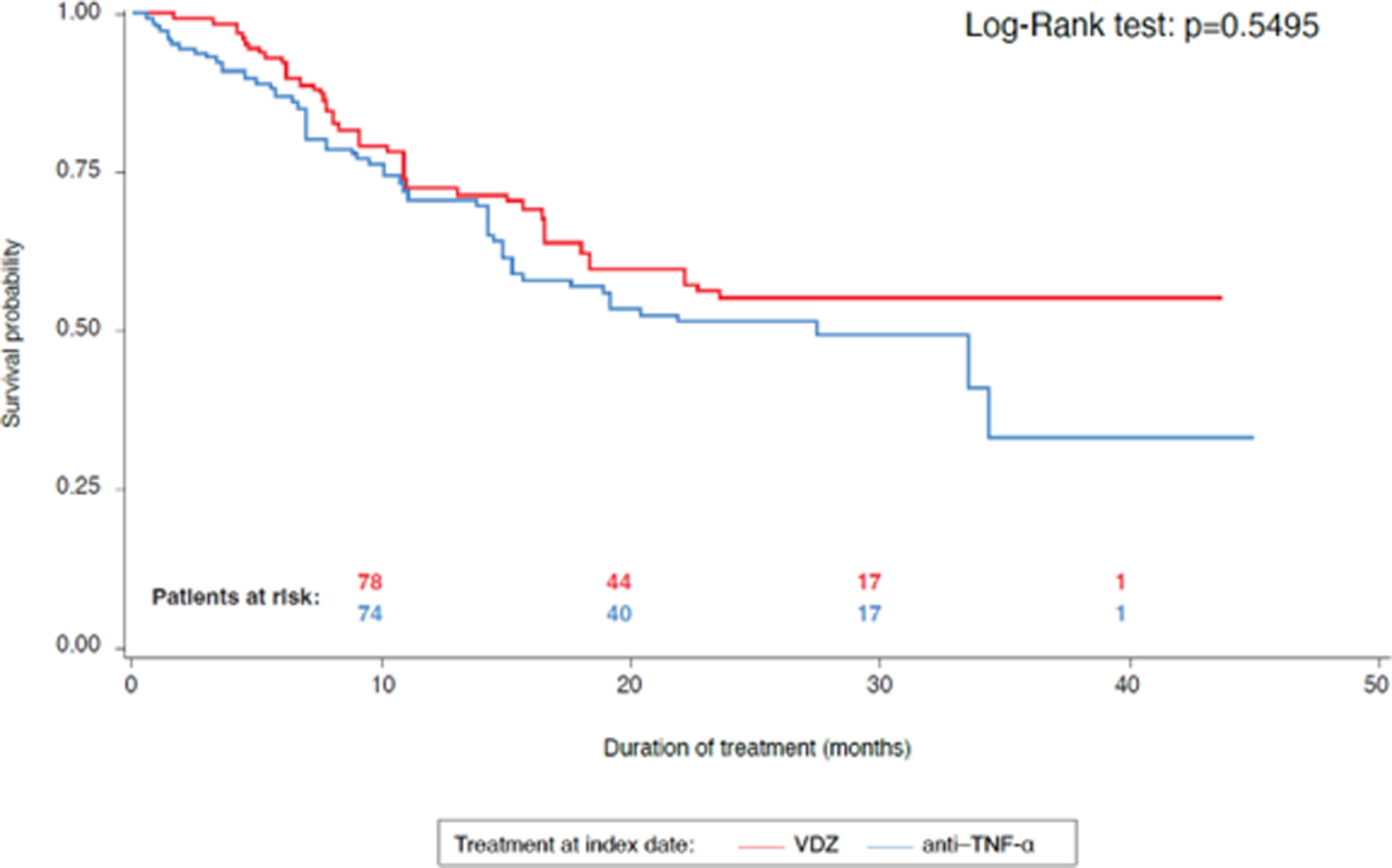
The rate of patients needing at least one treatment intensification, such as an increase in dose or dosing frequency, was 43.5% in VDZ and 39.5% in the anti-TNF-α cohort (p=0.586). Median time to first treatment intensification was 6.4 months in the VDZ cohort and 5.8 months in the anti-TNF-α cohort (p=0.967). The overall discontinuation rates for the whole follow-up were 39.6% with VDZ treatment and 50.0% with anti-TNF-α treatment (p=0.170). The probability of maintaining treatment was 79.0% and 76.3% at 10 months, 59.9% and 53.7% at 20 months, 55.1% and 33.5% at 45 months, in the VDZ cohort and the anti-TNF-α cohort, respectively (Fig. 6).
Healthcare resource utilizationSimilar patterns were observed in the utilization of healthcare resources for both the VDZ and anti-TNF-α cohorts.
The rates of patients visiting the emergency department at least once at 12 months post-index date were 15.7% in the VDZ cohort and 6.2% in the anti-TNF-α cohort.
The rates of patients that were hospitalized at least once at 12 months post-index date were 5.9% VDZ cohort and 9.3% in the anti-TNF-α cohort.
The quantification of other healthcare resource utilization is shown in Table S2.
DiscussionThe EVOLVE-IBERIA study aimed to describe the RWE of clinical outcomes achieved by patients with UC with no or limited exposure to prior biologic treatments.
To date, RWE data on VDZ has focused on highly treatment-refractory cohorts. A RWE European study of patients with IBD published in 2018, reported that 79.1% of patients responded to treatment by Week 14, including 39.5% that were in clinical remission.2 A recent study published in 2023 found that clinical remission among patients with UC was relatively low and similar in VDZ- and anti-TNF-α-treated patients and that clinical remission rates after 2 years were significantly higher for VDZ-treated patients than those treated with an anti-TNF-α agent.21
In this study, clinical response rates were similar in both cohorts at Week 14 and Week 52. Clinical remission improved similarly from Week 14 to Week 52, suggesting comparable long-term effectiveness with both treatments.
Biochemical remission rates at 52 weeks (based on fecal calprotectin<250μ/g) were also comparable in the VDZ cohort and the anti-TNF-α.
Endoscopic remission rates at Week 52 were comparable in both cohorts. These particular findings are significant, given that signs of endoscopic disease activity might persist even during clinical remission, and endoscopic remission can be considered as a more reliable predictor of favorable clinical outcomes.22
The VDZ cohort showed a significantly lower rate of treatment-related AEs versus the anti-TNF-α cohort (p=0.037). AEs of special interest (infections, serious infections, and malignancies) were higher in the anti-TNF-α cohort (p=0.008). Therefore, safety data suggest a favorable safety profile for VDZ compared with anti-TNF-α.
Time to treatment intensification and to treatment discontinuation resulted similar in both cohorts. Additionally, treatment persistence was higher at 45 months for the VDZ cohort compared with the anti-TNF-α cohort. Non-persistence of treatment in IBD is associated with increased overall costs and to a higher burden of disease for patients.23
The EVOLVE-IBERIA study (NCT03710486) supports the RWE findings of a previous EVOLVE study conducted in Canada, Greece, and the USA,19 demonstrating that first-line VDZ and anti-TNF-α therapies achieve similar clinical effectiveness rates up to 2 years post-treatment initiation, with VDZ showing a favorable benefit–risk profile as a first-line biologic therapy for patients with IBD.
Study limitationsThis was a retrospective study, so that its observational nature could have unbalanced the baseline covariates affecting the outcomes. To minimize this risk, the baseline covariates were balanced between both cohorts by means of PS-IPTW.
The use of both the partial Mayo score and physician judgment to evaluate clinical response introduces potential bias, as physician assessments can vary based on subjective interpretation and clinical experience. This is a common issue in retrospective studies, where medical records may differ in how information is documented. Recognizing this bias is essential for accurately interpreting the study results and applying them in clinical practice.
One of the main limitations of our study is the restricted availability of endoscopic data. Colonoscopies were not routinely performed during follow-up, as they were typically reserved for patients with suboptimal clinical response or suspicion of ongoing disease activity. As a result, the number of patients with available endoscopic assessments was limited. While both groups were clinically comparable within this subset, these data likely reflect outcomes in poorer responders and may not be generalizable to the broader study population. This selection bias should be considered when interpreting endoscopic outcomes.
As with any RWE study, the accuracy and completeness of the medical records available would have an impact on the quality of the data obtained in the study.
ConclusionsData from the EVOLVE-IBERIA study demonstrate the similar long-term effectiveness of VDZ and anti-TNF-α as first- or second-line biologic treatments of UC. Additionally, treatment with VDZ demonstrated a favorable safety profile compared with anti-TNF-α.
Author contributionsFC and FM were involved in the study design, conceptualization, methodology, patient recruitment, data interpretation, and reviewing drafts.
AG-C, CR, JPG, SR, CHG, IV-M, MPMM, SB, and IM were involved in patient recruitment, data interpretation, and reviewing drafts.
IT, JA were involved in project administration, methodology, data interpretation, and reviewing drafts.
TLA was involved in project administration, data interpretation, and reviewing drafts.
CM was involved in the study design, conceptualization, methodology, data interpretation, and reviewing drafts.
All authors agree with the manuscript's results and conclusions and approved the final version.
Ethical considerationsThe EVOLVE-IBERIA study (NCT03710486) was conducted in accordance with the ethical principles derived from the Declaration of Helsinki, Good Clinical Practice (GCP), the International Society for Pharmacoepidemiology (ISPE), Guidelines for Good Pharmacoepidemiology Practice (GPP), and any applicable local regulations.
FundingThis study was funded by Takeda Farmacéutica España, S.A.
Conflicts of interestFC: Research funding from AbbVie, Ferring, MSD, Shire, Takeda, and Zambon; speaker fees from AbbVie, Chiesi, Ferring, Gebro, MSD, Shire, Takeda, and Zambon.
FM: Research funding from Janssen, speaker fees from AbbVie, Ferring, Faes, Takeda and Pfizer, advisory board from Janssen, AbbVie, and Galapagos.
AGC: Has served as speaker, consultant, and advisory member for Janssen, AbbVie, Takeda, Pfizer, MSD, Khern, Galapagos, Ferring, FAES, Adacyte, Sandoz, Tillots.
CR: Has served as speaker, consultant, and/or advisory member for Janssen, AbbVie, Takeda, Pfizer, MSD, Galapagos, Ferring, and Tillots.
JPG: Has served as speaker, consultant, and advisory member for or has received research funding from MSD, AbbVie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine, and Vifor Pharma.
SR: Has served as speaker, a consultant and advisory member for or has received research funding from Takeda, Janssen, MSD, AbbVie, Arena, Biogen, Pfizer, Kern Pharma, Faes Farma, Ferring, Tillots, Adacyte, Shire Pharmaceuticals, Dr. Falk.
CHG: Has received payment for presentations and advice from Takeda.
IVM: has served as a speaker, a consultant and advisory member for or has received research funding from AbbVie, Msd, Janssen, Takeda, Ferring, Pfizer, Falk, Vifor Pharma, Tillots Pharma.
MPMM: has served as speaker, consultant and advisory member for or has received research funding from MSD, AbbVie, Pfizer, Kern Pharma, Takeda, Janssen, Shire Pharmaceuticals, and Otsuka Pharmaceutical.
SB: speaker fees AbbVie and Janssen and research support AbbVie and Janssen, participation in clinical trials and presentations with AbbVie and Pfizer.
IM: Has no conflicts of interest to declare.
JA, IT, TLA and CM: Full-time employees in Takeda Farmacéutica España.
The authors would like to thank to the group of investigators participating in EVOLVE-IBERIA study.
Medical writing support for the development of this article was funded by Takeda Farmacéutica España SA, under the direction of the authors, was provided by Aldo Olivieri, PhD, DNA Communications, an IPG DXTRA company in compliance with Good Publication Practice 3 ethical guidelines (DeTora LM, et al. Ann Intern Med. 2022;175(9):1298–1304).